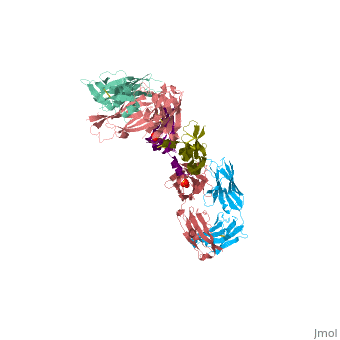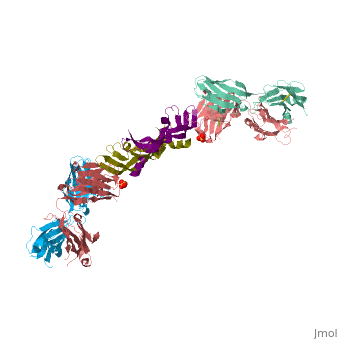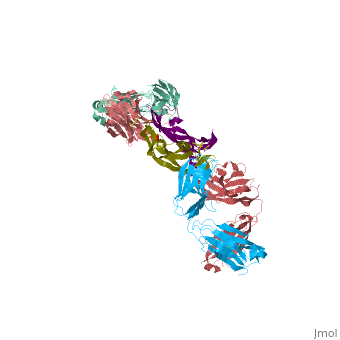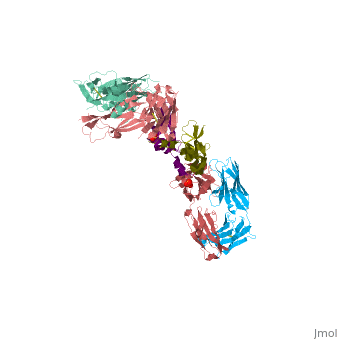VEGF IN COMPLEX WITH A NEUTRALIZING ANTIBODY
From Proteopedia
| (6 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | <StructureSection load='1bj1' size='340' side='right' caption='' scene=''> | + | <StructureSection load='1bj1' size='340' side='right' caption='Human VEGF receptor binding domain (dark olive, purple) complex with antibody (aqua, blue, red, rust) (PDB code [[1bj1]])' scene=''> |
| - | '''VEGF in complex with a neutralizing antibody'''<ref>PMID 9753694</ref>. | ||
== Backround == | == Backround == | ||
| Line 25: | Line 24: | ||
VEGF and anti-VEGF Fab Complex is a 6 chain, 1050 amino acid structure with a sequence taken from Homo sapiens and Mus musculus. | VEGF and anti-VEGF Fab Complex is a 6 chain, 1050 amino acid structure with a sequence taken from Homo sapiens and Mus musculus. | ||
For a guided tour on the structure components use[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1BJ1 FirstGlance] | For a guided tour on the structure components use[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1BJ1 FirstGlance] | ||
| - | VEGF is a <scene name='70/701451/Homodimeric_vegf/1'>homodimeric</scene> protein who belongs to the family of cystine knot growth factors. | + | VEGF is a <scene name='70/701451/Homodimeric_vegf/1'>homodimeric</scene> protein who belongs to the family of cystine knot growth factors <ref>PMID:9753694</ref> . |
while the 4 other chains belongs to the <scene name='70/701451/1bj1_fab_fragment_emphesys/1'>FAB fragment</scene>. | while the 4 other chains belongs to the <scene name='70/701451/1bj1_fab_fragment_emphesys/1'>FAB fragment</scene>. | ||
VEGF and anti-VEGF Fab Complex | VEGF and anti-VEGF Fab Complex | ||
The structure of the <scene name='70/701451/Receptor_binding_domain/1'>receptor-binding domain</scene> (residues 8–109) of VEGF consists of a central four- stranded β sheet that displays the characteristic cystine knot at one end and possesses a small hydrophobic core at the other end. | The structure of the <scene name='70/701451/Receptor_binding_domain/1'>receptor-binding domain</scene> (residues 8–109) of VEGF consists of a central four- stranded β sheet that displays the characteristic cystine knot at one end and possesses a small hydrophobic core at the other end. | ||
| + | |||
| + | ==3D structure of VEGF== | ||
| + | |||
| + | [[Vascular Endothelial Growth Factor]] | ||
</StructureSection> | </StructureSection> | ||
| Line 34: | Line 37: | ||
BACKGROUND: Vascular endothelial growth factor (VEGF) is a highly specific angiogenic growth factor; anti-angiogenic treatment through inhibition of receptor activation by VEGF might have important therapeutic applications in diseases such as diabetic retinopathy and cancer. A neutralizing anti-VEGF antibody shown to suppress tumor growth in an in vivo murine model has been used as the basis for production of a humanized version. RESULTS: We present the crystal structure of the complex between VEGF and the Fab fragment of this humanized antibody, as well as a comprehensive alanine-scanning analysis of the contact residues on both sides of the interface. Although the VEGF residues critical for antibody binding are distinct from those important for high-affinity receptor binding, they occupy a common region on VEGF, demonstrating that the neutralizing effect of antibody binding results from steric blocking of VEGF-receptor interactions. Of the residues buried in the VEGF-Fab interface, only a small number are critical for high-affinity binding; the essential VEGF residues interact with those of the Fab fragment, generating a remarkable functional complementarity at the interface. CONCLUSIONS: Our findings suggest that the character of antigen-antibody interfaces is similar to that of other protein-protein interfaces, such as ligand-receptor interactions; in the case of VEGF, the principal difference is that the residues essential for binding to the Fab fragment are concentrated in one continuous segment of polypeptide chain, whereas those essential for binding to the receptor are distributed over four different segments and span across the dimer interface. | BACKGROUND: Vascular endothelial growth factor (VEGF) is a highly specific angiogenic growth factor; anti-angiogenic treatment through inhibition of receptor activation by VEGF might have important therapeutic applications in diseases such as diabetic retinopathy and cancer. A neutralizing anti-VEGF antibody shown to suppress tumor growth in an in vivo murine model has been used as the basis for production of a humanized version. RESULTS: We present the crystal structure of the complex between VEGF and the Fab fragment of this humanized antibody, as well as a comprehensive alanine-scanning analysis of the contact residues on both sides of the interface. Although the VEGF residues critical for antibody binding are distinct from those important for high-affinity receptor binding, they occupy a common region on VEGF, demonstrating that the neutralizing effect of antibody binding results from steric blocking of VEGF-receptor interactions. Of the residues buried in the VEGF-Fab interface, only a small number are critical for high-affinity binding; the essential VEGF residues interact with those of the Fab fragment, generating a remarkable functional complementarity at the interface. CONCLUSIONS: Our findings suggest that the character of antigen-antibody interfaces is similar to that of other protein-protein interfaces, such as ligand-receptor interactions; in the case of VEGF, the principal difference is that the residues essential for binding to the Fab fragment are concentrated in one continuous segment of polypeptide chain, whereas those essential for binding to the receptor are distributed over four different segments and span across the dimer interface. | ||
| - | VEGF and the Fab fragment of a humanized neutralizing antibody: crystal structure of the complex at 2.4 A resolution and mutational analysis of the interface.,Muller YA, Chen Y, Christinger HW, Li B, Cunningham BC, Lowman HB, de Vos AM Structure. 1998 Sep 15;6(9):1153-67. PMID:9753694 | + | VEGF and the Fab fragment of a humanized neutralizing antibody: crystal structure of the complex at 2.4 A resolution and mutational analysis of the interface.,Muller YA, Chen Y, Christinger HW, Li B, Cunningham BC, Lowman HB, de Vos AM Structure. 1998 Sep 15;6(9):1153-67. PMID:9753694 |
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | ||
Current revision
| |||||||||||
Publication Abstract from PubMed
BACKGROUND: Vascular endothelial growth factor (VEGF) is a highly specific angiogenic growth factor; anti-angiogenic treatment through inhibition of receptor activation by VEGF might have important therapeutic applications in diseases such as diabetic retinopathy and cancer. A neutralizing anti-VEGF antibody shown to suppress tumor growth in an in vivo murine model has been used as the basis for production of a humanized version. RESULTS: We present the crystal structure of the complex between VEGF and the Fab fragment of this humanized antibody, as well as a comprehensive alanine-scanning analysis of the contact residues on both sides of the interface. Although the VEGF residues critical for antibody binding are distinct from those important for high-affinity receptor binding, they occupy a common region on VEGF, demonstrating that the neutralizing effect of antibody binding results from steric blocking of VEGF-receptor interactions. Of the residues buried in the VEGF-Fab interface, only a small number are critical for high-affinity binding; the essential VEGF residues interact with those of the Fab fragment, generating a remarkable functional complementarity at the interface. CONCLUSIONS: Our findings suggest that the character of antigen-antibody interfaces is similar to that of other protein-protein interfaces, such as ligand-receptor interactions; in the case of VEGF, the principal difference is that the residues essential for binding to the Fab fragment are concentrated in one continuous segment of polypeptide chain, whereas those essential for binding to the receptor are distributed over four different segments and span across the dimer interface.
VEGF and the Fab fragment of a humanized neutralizing antibody: crystal structure of the complex at 2.4 A resolution and mutational analysis of the interface.,Muller YA, Chen Y, Christinger HW, Li B, Cunningham BC, Lowman HB, de Vos AM Structure. 1998 Sep 15;6(9):1153-67. PMID:9753694
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
</div>