PLC beta 3 Gq
From Proteopedia
(Difference between revisions)
| (One intermediate revision not shown.) | |||
| Line 1: | Line 1: | ||
==Unique bidirectional interactions of Phospholipase C beta 3 with G alpha Q== | ==Unique bidirectional interactions of Phospholipase C beta 3 with G alpha Q== | ||
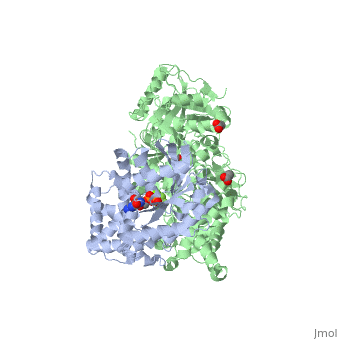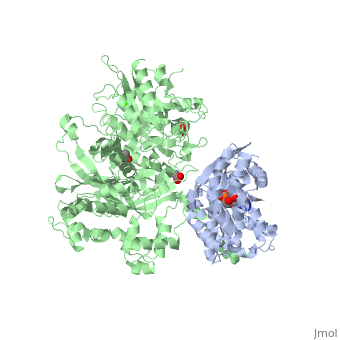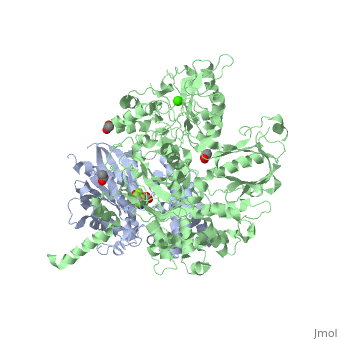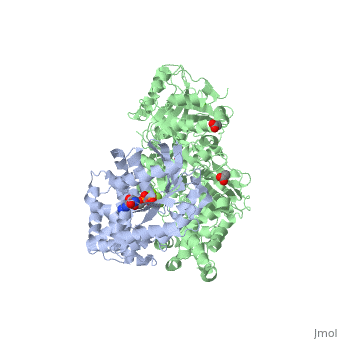
| - | <StructureSection load='3ohm' size='340' side='right' caption=' | + | <StructureSection load='3ohm' size='340' side='right' caption='Human phospholipase C β 3 (green) complex with guanine nucleotide-binding protein subunit α (grey) (PDB code [[3ohm]]) ' scene=''> |
== Introduction == | == Introduction == | ||
| - | Phospholipase C beta 3 ([http://en.wikipedia.org/wiki/PLCB3 PLC-β3]) catalyzes the hydrolysis of phosphatidylinositol 4,5 bisphosphate ([http://en.wikipedia.org/wiki/Phosphatidylinositol_4,5-bisphosphate PIP2]) to the second messengers inositol 1,4,5-trisphosphate ([http://en.wikipedia.org/wiki/Inositol_trisphosphate IP3]) and diacylglycerol ([http://en.wikipedia.org/wiki/Diglyceride DAG]) in an essential step for many physiological cascades. When the receptor stimulated by ligand of some kind, it increases exchange of guanosine diphosphate (GDP) to guanosine triphosphate (GTP) on [http://en.wikipedia.org/wiki/Gq_alpha_subunit Gαq]. GTP-bound Gαq activates PLC-β3, and PLC-β3 increases up to three orders of magnitude the rate of hydrolysis of GTP by its activating G protein. This is a unique mechanism in which PLC-β3 enzyme has the ability to terminate the Gαq protein signal, in addition to being activated by it | + | '''Phospholipase C beta 3''' ([http://en.wikipedia.org/wiki/PLCB3 PLC-β3]) catalyzes the hydrolysis of phosphatidylinositol 4,5 bisphosphate ([http://en.wikipedia.org/wiki/Phosphatidylinositol_4,5-bisphosphate PIP2]) to the second messengers inositol 1,4,5-trisphosphate ([http://en.wikipedia.org/wiki/Inositol_trisphosphate IP3]) and diacylglycerol ([http://en.wikipedia.org/wiki/Diglyceride DAG]) in an essential step for many physiological cascades. When the receptor stimulated by ligand of some kind, it increases exchange of guanosine diphosphate (GDP) to guanosine triphosphate (GTP) on [http://en.wikipedia.org/wiki/Gq_alpha_subunit Gαq]. GTP-bound Gαq activates PLC-β3, and PLC-β3 increases up to three orders of magnitude the rate of hydrolysis of GTP by its activating G protein. This is a unique mechanism in which PLC-β3 enzyme has the ability to terminate the Gαq protein signal, in addition to being activated by it<ref name="Waldo">PMID:20966218</ref><ref>PMID:23880553</ref>. |
== Structural highlights == | == Structural highlights == | ||
| Line 21: | Line 21: | ||
<scene name='70/701452/Fig7/1'>An extended loop (residues 254-268)</scene> between EF hands 3 and 4 of PLC-β3 interacts with the GTP-binding region of Gαq. Asn260 of the EF3/4 loop promotes GTP hydrolysis by interaction with the side chain of Gln209 of Gαq, which rearranges during GTP hydrolysis to stabilize the transition state mimicked by GDP•AlF4–•H20. Asn260 also interacts with Glu212 to stabilize switch 1 for GTP hydrolysis. | <scene name='70/701452/Fig7/1'>An extended loop (residues 254-268)</scene> between EF hands 3 and 4 of PLC-β3 interacts with the GTP-binding region of Gαq. Asn260 of the EF3/4 loop promotes GTP hydrolysis by interaction with the side chain of Gln209 of Gαq, which rearranges during GTP hydrolysis to stabilize the transition state mimicked by GDP•AlF4–•H20. Asn260 also interacts with Glu212 to stabilize switch 1 for GTP hydrolysis. | ||
| - | Other effectors are known to engage Gαq throughout <scene name='70/701452/Fig3/4'>different regions (pink)</scene>. In addition, there are a large family of regulator of G protein signaling (RGS) proteins that independently accelerates the GTP hydrolysis, these RGS proteins engage the GTPase domain throughout <scene name='70/701452/Fig4/4'>several residues (brown)</scene> on Gαq. PLC-β3 interacts with <scene name='70/701452/Fig5/5'>residues (blue)</scene> on Gαq that overlaps almost completely with portions of Gαq needed for engagement of RGS proteins and other effectors | + | Other effectors are known to engage Gαq throughout <scene name='70/701452/Fig3/4'>different regions (pink)</scene>. In addition, there are a large family of regulator of G protein signaling (RGS) proteins that independently accelerates the GTP hydrolysis, these RGS proteins engage the GTPase domain throughout <scene name='70/701452/Fig4/4'>several residues (brown)</scene> on Gαq. PLC-β3 interacts with <scene name='70/701452/Fig5/5'>residues (blue)</scene> on Gαq that overlaps almost completely with portions of Gαq needed for engagement of RGS proteins and other effectors<ref name="Waldo"/>. |
'''Critical residues in the interface:''' | '''Critical residues in the interface:''' | ||
Current revision
Unique bidirectional interactions of Phospholipase C beta 3 with G alpha Q
| |||||||||||
References
- ↑ 1.0 1.1 Waldo GL, Ricks TK, Hicks SN, Cheever ML, Kawano T, Tsuboi K, Wang X, Montell C, Kozasa T, Sondek J, Harden TK. Kinetic Scaffolding Mediated by a Phospholipase C-{beta} and Gq Signaling Complex. Science. 2010 Nov 12;330(6006):974-80. Epub 2010 Oct 21. PMID:20966218 doi:10.1126/science.1193438
- ↑ Lyon AM, Tesmer JJ. Structural insights into phospholipase C-beta function. Mol Pharmacol. 2013 Oct;84(4):488-500. doi: 10.1124/mol.113.087403. Epub 2013 Jul, 23. PMID:23880553 doi:http://dx.doi.org/10.1124/mol.113.087403