Sandbox myosinkinesin
From Proteopedia
(Difference between revisions)
(This page is setup for Spencer to build his senior project for OU CHEM 4923) |
|||
| (32 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | This | + | ==Kinesin== |
| + | <StructureSection load='4uxr' size='340' side='right' caption='This is the head region of kinesin' scene=''> | ||
| + | |||
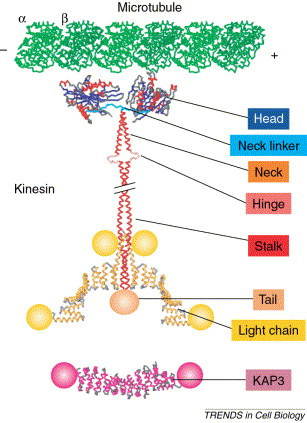
| + | [[Image:1-s2.0-S0962892402024005-gr1.jpg]] | ||
| + | This is all the parts of a Kinesin Dimer | ||
| + | |||
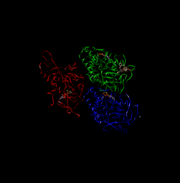
| + | [[Image:4UXR.png | thumb | This is 4UXR, the head of kinesin.]]<ref name= "BLAHHH">doi:10.1016/S0962-8924(02)02400-5</ref> | ||
| - | ==Your Heading Here (maybe something like 'Structure')== | ||
| - | <StructureSection load='1stp' size='340' side='right' caption='Caption for this structure' scene=''> | ||
| - | This is a default text for your page '''Sandbox myosinkinesin'''. Click above on '''edit this page''' to modify. Be careful with the < and > signs. | ||
| - | You may include any references to papers as in: the use of JSmol in Proteopedia <ref>DOI 10.1002/ijch.201300024</ref> or to the article describing Jmol <ref>PMID:21638687</ref> to the rescue. | ||
== Function == | == Function == | ||
| - | == | + | Kinesin is generally responsible for the transport of polypeptides and other large molecules throughout a single cell, such as moving the spindles during cell division. Kinesin can only move from the negative (-) end of microtubules to the positive (+) end. This means that kinesin only moves objects out towards the periphery of the cell and thus aids in the secretion of molecules or division of cells.<ref>doi: 10.2210/rcsb_pdb/mom_2005_4</ref> By binding to ATP this causes a conformational change that allows the head group to bind to the microtubule, which causes a pivot in the neck region and eventually drags the entire protein along the microtubule. <ref name= "NECK"/><ref name= "OP"/> |
| + | |||
| + | == Diseases == | ||
| + | Research is still being done, but possible medical problems associated with kinesin failure could include mitotic/meiotic diseases (Down syndrome, etc.), failure of cilia properly working could influence organs like the kidney (polycystic kidney disease), and neuropathy (Alzheimer, etc.). | ||
| + | <ref name= "BLAHHH"/> | ||
| + | |||
| - | == Relevance == | ||
== Structural highlights == | == Structural highlights == | ||
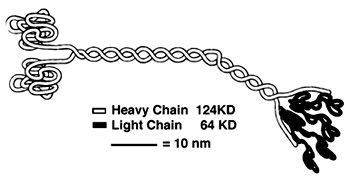
| - | + | [[Image:Hirokawa.jpg | Here is a picture of the light chains.]] <ref>Hackney, David. "Kinesin-1 Structure." Duke. Duke, 25 Jan. 2005. Web. 17 Dec. 2015. <https://labs.cellbio.duke.edu/kinesin/KinesinStructure.html>.</ref> | |
| + | '''''Heavy Chain:''''' | ||
| + | |||
| + | It is the most conserved region amongst kinesin which consists of the head, neck, and tail. Usually contains eight core β-sheets and six major alpha helixes, most of these secondary structures are in different places in the primary sequence but line up in the tertiary structure.<ref name= "OP"/> | ||
| + | '''''Light Chain:''''' | ||
| + | Not technically part of the protein of kinesin or myosin itself, but its presence is necessary for activity. It regulates conformational changes within the protein. Is usually alpha helical in structure. <ref> PMID:8316857</ref> | ||
| + | |||
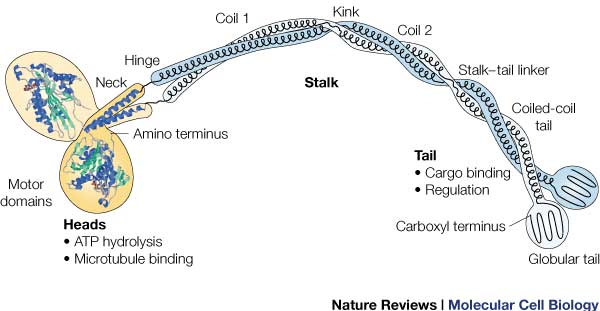
| + | [[Image:131631646354632.jpg | This is the overall structure of a functional kinesin dimer. ]] | ||
| + | |||
| + | |||
| + | '''Head:''' | ||
| + | Most conserved domain amongst all kinesin, it consists mainly of alpha helixes. Its tertiary structure usually includes a large cleft from the actin binding site to the ATP binding pocket. The head has the ability to bind microtubule on one site and bind ATP at another. This section undergoes the most conformational change and is responsible for the force that causes kinesin to move along the cytoskeleton. In kinesin, binding of ATP appears to have allosteric control over the binding of kinesin to the tubules, by a twisting of a β- sheet core, showing allosteric control between the subdomains within the protein. <ref>DOI: 10.1039/c3cp53367k</ref> | ||
| + | |||
| + | ATPase: It generates this force using an ATPase either coupled with the myosin/kinesin or inherent within the head, the inherent ATPase uses a P-loop, which is a phosphate- binding loop used in adenylate kinase, Ras, and others. Both also create a similar environment for the γ-phosphate, which uses a conserve motif of Ser-Ser-Arg.<ref name= "RAS">doi: 10.1038/380550a0</ref> | ||
| + | |||
| + | In kinesin, the binding of ATP greatly increases the binding of kinesin to the microtubules, to allow it to perform a power stroke.<ref name= "OP"/> | ||
| + | ''RAS fold'': conserved domain among many signaling proteins. A structural motif where a nucleotide molecule is bound to loops at one end of a β-sheet domain. This suggests that myosin and kinesin may have evolved from a common ancestor.<ref name= "RAS"/> | ||
| + | |||
| + | |||
| + | '''Neck:''' | ||
| + | |||
| + | Completely alpha-helical region between the head and the tail on the heavy chain that binds to the light chains. <ref name= "NECK">doi:10.1038/45483</ref> In kinesin, the neck region of amino acids 330-370 generally form a coiled coil region that is long and flexible.<ref name= "OP">doi: 10.1093/emboj/20.22.6213</ref> It transforms the conformational changes within the head into movement along the microtubules<ref name= "NECK"/>. | ||
| + | |||
| + | |||
| + | '''Tail:''' | ||
| + | |||
| + | Includes polypeptide binding site, varies the most since it is responsible for dictating the location within the cell that the protein is active. <ref name= "OP"/> | ||
</StructureSection> | </StructureSection> | ||
| + | |||
== References == | == References == | ||
| + | |||
| + | |||
| + | |||
<references/> | <references/> | ||
Current revision
Kinesin
| |||||||||||
References
- ↑ 1.0 1.1 Mandelkow E, Mandelkow EM. Kinesin motors and disease. Trends Cell Biol. 2002 Dec;12(12):585-91. doi: 10.1016/s0962-8924(02)02400-5. PMID:12495847 doi:http://dx.doi.org/10.1016/s0962-8924(02)02400-5
- ↑ doi: https://dx.doi.org/10.2210/rcsb_pdb/mom_2005_4
- ↑ 3.0 3.1 3.2 Rice S, Lin AW, Safer D, Hart CL, Naber N, Carragher BO, Cain SM, Pechatnikova E, Wilson-Kubalek EM, Whittaker M, Pate E, Cooke R, Taylor EW, Milligan RA, Vale RD. A structural change in the kinesin motor protein that drives motility. Nature. 1999 Dec 16;402(6763):778-84. PMID:10617199 doi:http://dx.doi.org/10.1038/45483
- ↑ 4.0 4.1 4.2 4.3 4.4 Song YH, Marx A, Muller J, Woehlke G, Schliwa M, Krebs A, Hoenger A, Mandelkow E. Structure of a fast kinesin: implications for ATPase mechanism and interactions with microtubules. EMBO J. 2001 Nov 15;20(22):6213-25. PMID:11707393 doi:10.1093/emboj/20.22.6213
- ↑ Hackney, David. "Kinesin-1 Structure." Duke. Duke, 25 Jan. 2005. Web. 17 Dec. 2015. <https://labs.cellbio.duke.edu/kinesin/KinesinStructure.html>.
- ↑ Rayment I, Rypniewski WR, Schmidt-Base K, Smith R, Tomchick DR, Benning MM, Winkelmann DA, Wesenberg G, Holden HM. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50-8. PMID:8316857
- ↑ Krukau A, Knecht V, Lipowsky R. Allosteric control of kinesin's motor domain by tubulin: a molecular dynamics study. Phys Chem Chem Phys. 2014 Apr 7;16(13):6189-98. doi: 10.1039/c3cp53367k. PMID:24561904 doi:http://dx.doi.org/10.1039/c3cp53367k
- ↑ 8.0 8.1 Kull FJ, Sablin EP, Lau R, Fletterick RJ, Vale RD. Crystal structure of the kinesin motor domain reveals a structural similarity to myosin. Nature. 1996 Apr 11;380(6574):550-5. PMID:8606779 doi:http://dx.doi.org/10.1038/380550a0