Arginine Kinase AK
From Proteopedia
(Difference between revisions)
| (6 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
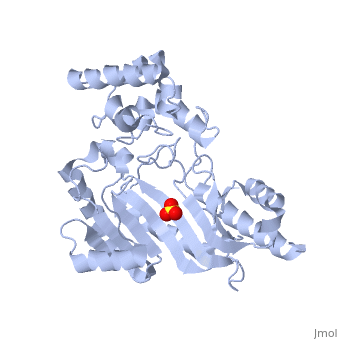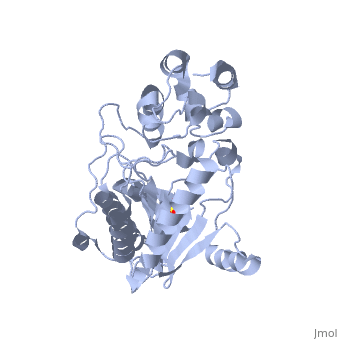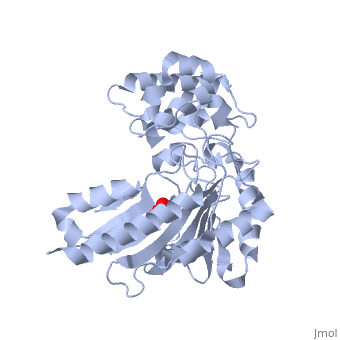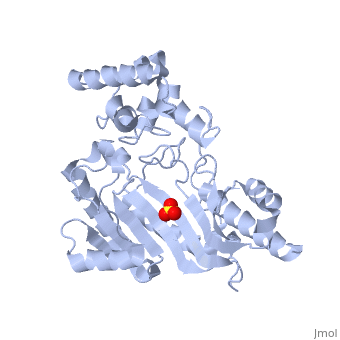
| - | < | + | <StructureSection load='3M10' size='350' side='right' scene='' caption='The 3D structure of a bound confirmation of Arginine Kinase complex with sulfate (PDB code [[3m10]])'> |
| + | |||
==Arginine Kinase== | ==Arginine Kinase== | ||
A phosphokinase used to store energy in the form of Argininephosphate. | A phosphokinase used to store energy in the form of Argininephosphate. | ||
| Line 9: | Line 10: | ||
Developmental Biology, Swiss Federal Institute of Technology, Zurich, 21 June 1973. Web. 12 Nov. 2015</ref>. This is done by the transfer of an N-phosphoryl group from phosphocreatine to ADP. | Developmental Biology, Swiss Federal Institute of Technology, Zurich, 21 June 1973. Web. 12 Nov. 2015</ref>. This is done by the transfer of an N-phosphoryl group from phosphocreatine to ADP. | ||
== Structure == | == Structure == | ||
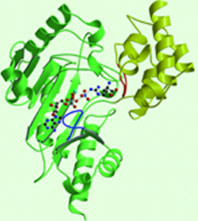
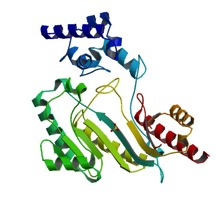
| - | The structure of <scene name='71/716599/My3/1'>arginine kinase</scene> is mainly α-helical and contains an N-terminal region with a specificity loop for specific substrate binding. (Figure 2). However, when compared to creatine kinase, arginine kinase is not terminated at the N-terminal end with a pair of proline-glycine residues. Typically within creatine kinase, the proline molecules restrict changes in conformation and is the amino acid that terminates helices. The glycine chains are usually associated with flexibility. However, in arginine kinase this is typically not the case. On the C-terminal end, there is an eight-stranded antiparallel <scene name='71/716599/Beta/1'>β-sheet</scene> with seven <scene name='71/716599/Mynewscene/1'>α-helices</scene> flanking the sheet (Figure 1). Residue 330 is an arginine that appears to play a crucial role in maintaining structural stability. Studies show that a mutation in the residue leads to a steep decline in enzymatic activity <ref>DOI 10.1016/j.ijbiomac.2012.12.015</ref>. | + | The structure of <scene name='71/716599/My3/1'>arginine kinase</scene> is mainly α-helical and contains an N-terminal region with a specificity loop for specific substrate binding. (Figure 2). However, when compared to creatine kinase, arginine kinase is not terminated at the N-terminal end with a pair of proline-glycine residues. Typically within creatine kinase, the proline molecules restrict changes in conformation and is the amino acid that terminates helices. The glycine chains are usually associated with flexibility. However, in arginine kinase this is typically not the case. On the C-terminal end, there is an eight-stranded antiparallel <scene name='71/716599/Beta/1'>β-sheet</scene> with seven <scene name='71/716599/Mynewscene/1'>α-helices</scene> flanking the sheet (Figure 1). Residue <scene name='71/716599/330/2'>330</scene> is an arginine that appears to play a crucial role in maintaining structural stability. Studies show that a mutation in the residue leads to a steep decline in enzymatic activity <ref>DOI 10.1016/j.ijbiomac.2012.12.015</ref>. |
[[Image:F1.large.jpg|left|frame|none|alt=Alt text|Figure 1. Structure of an AK in substrate-bound form<ref>http://www.pnas.org/content/95/15/8449/F1.large.jpg</ref>]] | [[Image:F1.large.jpg|left|frame|none|alt=Alt text|Figure 1. Structure of an AK in substrate-bound form<ref>http://www.pnas.org/content/95/15/8449/F1.large.jpg</ref>]] | ||
| - | + | {{Clear}} | |
| + | The small domain specificity loop is comprised of residues 61-68. The large domain specificity loop is comprised of residues 311-319. In this region, five residues differ between arginine and creatine kinases: 312, 314, 315, 317, and 319 <ref>Newsholme, E. A., Beis, I., Leech, A. R., & Zammit, V. A. (1978). The role of creatine kinase and arginine kinase in muscle. Biochemical Journal, 172(3), 533–537.</ref>. Within each arginine kinase, there is typically a Mg+2 ion adjacent to the antiparallel β-sheet (Figure 2) to aid in the increase of ATP’s affinity for the binding site <ref>DOI 10.1080/08927022.2011.561430</ref>. Typically two arginine kinase structures mirror each other and form a hole like structure in between the two (Figure 1). | ||
[[Image:3M10 bio r 500.jpg|inline|left|frame|none|alt=Alt text|Figure 2. Structure of an AK in an unbound conformation<ref>http://www.rcsb.org/pdb/explore.do?structureId=4GVZ</ref>]] | [[Image:3M10 bio r 500.jpg|inline|left|frame|none|alt=Alt text|Figure 2. Structure of an AK in an unbound conformation<ref>http://www.rcsb.org/pdb/explore.do?structureId=4GVZ</ref>]] | ||
| - | + | {{Clear}} | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
== Function == | == Function == | ||
Arginine Kinase is part of a class of kinases that regulates ATP levels in the body to help maintain homeostasis. Arginine Kinase is a phosphokinase - a kinase used in the catalyzation of phosphagens and adenosine diphosphate (ADP) into adenosine triphosphate (ATP). It creates a sort of storage option for energy. Phosphagens act as a storage form of phosphate (Nω-phospho-L-arginine) that can be catalyzed into an energy source (ATP) when needed <ref name=pereira2000> Pereira, C. A., Alonso, G. D., Paveto, M. C., Iribarren, A., Cabanas, M. L., Torres, H. N. | Arginine Kinase is part of a class of kinases that regulates ATP levels in the body to help maintain homeostasis. Arginine Kinase is a phosphokinase - a kinase used in the catalyzation of phosphagens and adenosine diphosphate (ADP) into adenosine triphosphate (ATP). It creates a sort of storage option for energy. Phosphagens act as a storage form of phosphate (Nω-phospho-L-arginine) that can be catalyzed into an energy source (ATP) when needed <ref name=pereira2000> Pereira, C. A., Alonso, G. D., Paveto, M. C., Iribarren, A., Cabanas, M. L., Torres, H. N. | ||
| Line 29: | Line 27: | ||
Arginine Kinase (AK) is represented by a single gene and the sequence or partial sequence is available from Drosophila, Limulus, lobster, shrimp, and abalone <ref>Wang, Yu-mei E., Pia Esbensen, and David Bentley. "Arginine Kinase Expression and | Arginine Kinase (AK) is represented by a single gene and the sequence or partial sequence is available from Drosophila, Limulus, lobster, shrimp, and abalone <ref>Wang, Yu-mei E., Pia Esbensen, and David Bentley. "Arginine Kinase Expression and | ||
Localization in Growth Cone Migration." The Journal of Neuroscience 18.3 | Localization in Growth Cone Migration." The Journal of Neuroscience 18.3 | ||
| - | (1998): 987-98. Web. 15 Nov. 2015. <http://www.jneurosci.org/content/18/3/987.full.pdf></ref>. In the study by Wang et al. 1998, a phylogenetic tree was put together for the evolution of Arginine Kinase compared to Creatine Kinase. It indicates that there are major clusters corresponding to Creatine Kinase and Arginine Kinase. Also, it is expressed in the gills of two species of euryhaline crabs, the blue crab Callinectes sapidus and the shore crab Carcinus maenus, in which energy-requiring functions include monovalent ion transport, acid-base balance, nitrogen excretion <ref>Kotlyar, S., Weihrauch, D., Paulsen, R., & Towle, D. (2000, July 20). Expression of Arginine Kinase Enzymatic Activity and mRNA in Gills of the Euryhaline Crabs Carcinus Maenas and Callinectes Sapidus. Retrieved November 17, 2015, from http://jeb.biologists.org/content/jexbio/203/16/2395.full.pdf</ref>. | + | (1998): 987-98. Web. 15 Nov. 2015. <http://www.jneurosci.org/content/18/3/987.full.pdf></ref>. In the study by Wang et al. 1998, a phylogenetic tree was put together for the evolution of Arginine Kinase compared to Creatine Kinase. It indicates that there are major clusters corresponding to Creatine Kinase and Arginine Kinase. Also, it is expressed in the gills of two species of euryhaline crabs, the blue crab Callinectes sapidus and the shore crab Carcinus maenus, in which energy-requiring functions include monovalent ion transport, acid-base balance, nitrogen excretion <ref>Kotlyar, S., Weihrauch, D., Paulsen, R., & Towle, D. (2000, July 20). Expression of Arginine Kinase Enzymatic Activity and mRNA in Gills of the Euryhaline Crabs Carcinus Maenas and Callinectes Sapidus. Retrieved November 17, 2015, from http://jeb.biologists.org/content/jexbio/203/16/2395.full.pdf</ref>. |
| + | </StructureSection> | ||
| + | == 3D structure of arginine kinase == | ||
| + | |||
| + | See [[Arginine kinase]] | ||
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
| |||||||||||
3D structure of arginine kinase
See Arginine kinase
References
- ↑ Strong, S., & Ellington, W. (1994). Isolation and sequence analysis of the gene for arginine kinase from the chelicerate arthropod, Limulus polyphemus: Insights into catalytically important residues. Biochimica Et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology, 197-200.
- ↑ 2.0 2.1 2.2 2.3 Wallimann, Theo, and Hans M. Eppenberger. "Properties of Arginine Kinase from Drosophila Melanogaster." Www.onlinelibrary.wiley.com. Laboratory of Developmental Biology, Swiss Federal Institute of Technology, Zurich, 21 June 1973. Web. 12 Nov. 2015
- ↑ Wang WD, Wang JS, Shi YL, Zhang XC, Pan JC, Zou GL. Mutation of residue arginine 330 of arginine kinase results in the generation of the oxidized form more susceptible. Int J Biol Macromol. 2013 Mar;54:238-43. doi: 10.1016/j.ijbiomac.2012.12.015., Epub 2012 Dec 19. PMID:23262386 doi:http://dx.doi.org/10.1016/j.ijbiomac.2012.12.015
- ↑

- ↑ Newsholme, E. A., Beis, I., Leech, A. R., & Zammit, V. A. (1978). The role of creatine kinase and arginine kinase in muscle. Biochemical Journal, 172(3), 533–537.
- ↑ doi: https://dx.doi.org/10.1080/08927022.2011.561430
- ↑ http://www.rcsb.org/pdb/explore.do?structureId=4GVZ
- ↑ 8.0 8.1 Pereira, C. A., Alonso, G. D., Paveto, M. C., Iribarren, A., Cabanas, M. L., Torres, H. N. & Flawia, M. M. (2000) Trypanosoma cruzi arginine kinase characterization and cloning., J. Biol.Chem. 275, 1495-1501.
- ↑ Azzi A, Clark SA, Ellington WR, Chapman MS. The role of phosphagen specificity loops in arginine kinase. Protein Sci. 2004 Mar;13(3):575-85. PMID:14978299 doi:10.1110/ps.03428304
- ↑ Wang, Yu-mei E., Pia Esbensen, and David Bentley. "Arginine Kinase Expression and Localization in Growth Cone Migration." The Journal of Neuroscience 18.3 (1998): 987-98. Web. 15 Nov. 2015. <http://www.jneurosci.org/content/18/3/987.full.pdf>
- ↑ Kotlyar, S., Weihrauch, D., Paulsen, R., & Towle, D. (2000, July 20). Expression of Arginine Kinase Enzymatic Activity and mRNA in Gills of the Euryhaline Crabs Carcinus Maenas and Callinectes Sapidus. Retrieved November 17, 2015, from http://jeb.biologists.org/content/jexbio/203/16/2395.full.pdf