User:Coline Perrin/CCL2
From Proteopedia
(New page: ==Introduction== <StructureSection load='1stp' size='340' side='right' caption='Caption for this structure' scene=''> The''' chemokine (C-C motif) ligand''' (CCL2), also known as : - monoc...) |
|||
| (2 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | + | <StructureSection load='1dok' size='350' side='right' caption='Monocyte chemoattractant protein 1 (PDB code [[3idf]])' scene='72/721520/Cv/2'> | |
| - | <StructureSection load=' | + | == Function and Structure == |
| - | The''' chemokine (C-C motif) ligand''' | + | |
| - | - | + | '''Human synthetic monocyte chemoattractant protein 1 (MCP)''' belongs to the superfamily of chemokines, which are proteins involved in immunoregulatory and inflammatory processes. The gene for CCL2 is on chromosome 17 in region 17q11.2-q12. The superfamily can be subdivided into 4 smaller groups, depending on the N-ter arangment of the cysteines. The CCL2<ref>PMID:8170963</ref> is also known as '''chemokine (C-C motif) ligand''' or: |
| + | - MCP1 | ||
- small inducible cytokine A2 (SCYA2) | - small inducible cytokine A2 (SCYA2) | ||
- MCAF | - MCAF | ||
| Line 11: | Line 12: | ||
- GDCF-2 | - GDCF-2 | ||
- HC11. | - HC11. | ||
| - | == Function == | ||
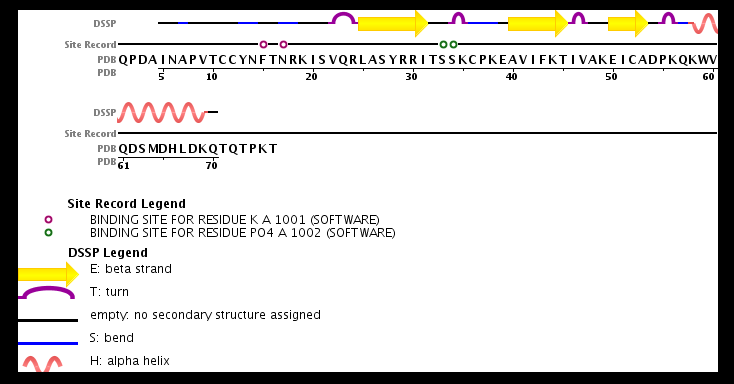
| - | == | + | It exists as a monomer or a dimer, eventhough the homodimer form is preferred. |
| + | The structure of the monomer is made of <scene name='72/721520/Ligand_binding_on_ccl2/2'>3 Beta sheets and 1 alpha helix</scene>. | ||
| + | == Ligands == | ||
| + | The known ligands for CCL2 are <scene name='72/721520/Ligand_binding_on_ccl2/1'>Potassium and PO4</scene>. The potassium binds to the S33 and S34 of the monomer and PO4 binds to F15 and N17. | ||
| - | == | + | == Evolutionary Conservation == |
| + | [[Image:Consurf_key_small.gif|200px|right]] | ||
| + | Check<jmol> | ||
| + | <jmolCheckbox> | ||
| + | <scriptWhenChecked>select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/if/3ifd_consurf.spt"</scriptWhenChecked> | ||
| + | <scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | ||
| + | <text>to colour the structure by Evolutionary Conservation</text> | ||
| + | </jmolCheckbox> | ||
| + | </jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. | ||
| + | <div style="clear:both"></div> | ||
| + | <div style="background-color:#fffaf0;"> | ||
| + | == Diseases == | ||
| + | |||
| + | CCL2 is implicated in several diseases like psoriasis and rheumatoid arthritis where the appear to recruit macrophages, therefore bolstering the inflammation on joints. | ||
| + | It is thought to be involved in atherosclerosis in the recruitment of monocytes into the arterial wall as well as in prostate cancer<ref>PMID:25917126</ref>. | ||
| + | It has also been found elevated in the urine of people with lupus as a sign warning of inflammation of the kidney. | ||
| + | CCL2 is overexpressed in epilepsy, brain ischemia, Alzheimer's disease, EAE and traumatic brain injury. | ||
== Structural highlights == | == Structural highlights == | ||
| - | + | CCL2 is part of the C-C motif group because of the covalent bond made between <scene name='72/721520/Cv/4'>2 of the 4 cysteines of the N terminal domain</scene>.<ref>PMID:8989326</ref> | |
| - | + | Post translational modifications at the N-terminus can regulate receptor and target cell selectivity. Deletion of the N-terminal residue converts it from an activator of basophil to an eosinophil chemoattractant. | |
</StructureSection> | </StructureSection> | ||
| + | |||
| + | [[Image:chaine.png]] | ||
| + | |||
| + | == Synthesis == | ||
| + | |||
| + | The protein human CCL2 has been synthesized using a combination of solid phase peptide synthesis (SPPS) and native chemical ligation (NCL). The thioester-peptide segment was synthesized using the sulfonamide safety-catch linker and 9-fluorenylmethoxycarbonyl (Fmoc) SPPS, and pseudoproline dipeptides were used to facilitate the synthesis of both CCL2 fragments. After assembly of the full-length peptide chain by NCL, a glutathione redox buffer was used to fold and oxidize the CCL2 protein. | ||
| + | CCL2 was crystallized and the structure was determined by X-ray diffraction at 1.9-A resolution. This is probably one of the first crystal structures of a protein prepared using the sulfonamide safety-catch linker and NCL. | ||
| + | |||
| + | ==3D structures of Monocyte chemoattractant protein== | ||
| + | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| + | {{#tree:id=OrganizedByTopic|openlevels=0| | ||
| + | |||
| + | * Monocyte chemoattractant protein 1 | ||
| + | |||
| + | **[[1dok]], [[1dol]] – hMCP-1 (mutant) - human<br /> | ||
| + | **[[3ifd]] – hMCP-1 <br /> | ||
| + | **[[1dom]], [[1don]] – hMCP-1 - NMR<br /> | ||
| + | **[[1ml0]], [[2nz1]] – hMCP-1 (mutant) + M3 protein<br /> | ||
| + | **[[2bdn]] – hMCP-1 + antibody<br /> | ||
| + | **[[4dn4]] – hMCP-1 (mutant) + antibody<br /> | ||
| + | **[[4zk9]] – hMCP-1 + chemokine binding protein<br /> | ||
| + | **[[4r8i]] – hMCP-1 + RNA<br /> | ||
| + | |||
| + | * Monocyte chemoattractant protein 2 | ||
| + | |||
| + | **[[1esr]] – hMCP-2 (mutant) <br /> | ||
| + | |||
| + | * Monocyte chemoattractant protein 3 | ||
| + | |||
| + | **[[1ncv]], [[1bo0]] – hMCP-3 - NMR<br /> | ||
| + | **[[4zkc]] – hMCP-3 + chemokine binding protein<br /> | ||
| + | |||
| + | * Monocyte chemoattractant protein 4 | ||
| + | |||
| + | **[[2ra4]] – hMCP-4 <br /> | ||
| + | }} | ||
== References == | == References == | ||
| + | https://fr.wikipedia.org/wiki/CCL2 | ||
| + | http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=1DOK | ||
| + | http://www.uniprot.org/uniprot/P13500#interaction | ||
| + | http://www.rcsb.org/pdb/explore/explore.do?structureId=3IFD | ||
<references/> | <references/> | ||
Current revision
| |||||||||||
Synthesis
The protein human CCL2 has been synthesized using a combination of solid phase peptide synthesis (SPPS) and native chemical ligation (NCL). The thioester-peptide segment was synthesized using the sulfonamide safety-catch linker and 9-fluorenylmethoxycarbonyl (Fmoc) SPPS, and pseudoproline dipeptides were used to facilitate the synthesis of both CCL2 fragments. After assembly of the full-length peptide chain by NCL, a glutathione redox buffer was used to fold and oxidize the CCL2 protein. CCL2 was crystallized and the structure was determined by X-ray diffraction at 1.9-A resolution. This is probably one of the first crystal structures of a protein prepared using the sulfonamide safety-catch linker and NCL.
3D structures of Monocyte chemoattractant protein
Updated on 27-January-2016
References
https://fr.wikipedia.org/wiki/CCL2 http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=1DOK http://www.uniprot.org/uniprot/P13500#interaction http://www.rcsb.org/pdb/explore/explore.do?structureId=3IFD
- ↑ Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3652-6. PMID:8170963
- ↑ Ito Y, Ishiguro H, Kobayashi N, Hasumi H, Watanabe M, Yao M, Uemura H. Adipocyte-derived monocyte chemotactic protein-1 (MCP-1) promotes prostate cancer progression through the induction of MMP-2 activity. Prostate. 2015 Jul 1;75(10):1009-19. doi: 10.1002/pros.22972. Epub 2015 Apr 27. PMID:25917126 doi:http://dx.doi.org/10.1002/pros.22972
- ↑ Lubkowski J, Bujacz G, Boque L, Domaille PJ, Handel TM, Wlodawer A. The structure of MCP-1 in two crystal forms provides a rare example of variable quaternary interactions. Nat Struct Biol. 1997 Jan;4(1):64-9. PMID:8989326