Glut3
From Proteopedia
(Difference between revisions)
| (4 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
==Facilitated Glucose Transporter 3, Solute Carrier Family 2 (GLUT3/ SLC2A3) in Homo Sapiens== | ==Facilitated Glucose Transporter 3, Solute Carrier Family 2 (GLUT3/ SLC2A3) in Homo Sapiens== | ||
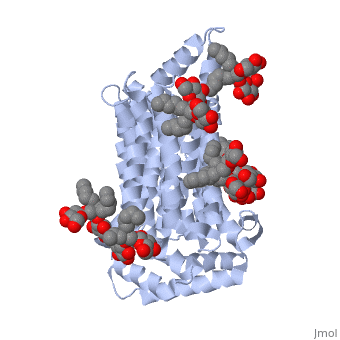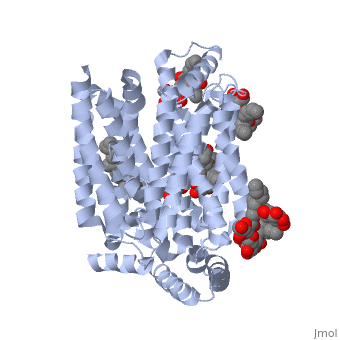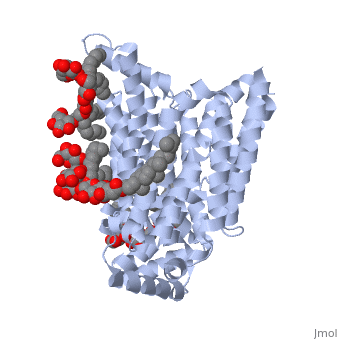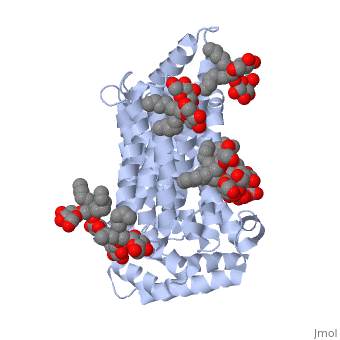
| - | <StructureSection load='5c65' size='340' side='right' caption='Human glucose transporter complex with cholesterol derivative (PDB code [[5c65]]) | + | <StructureSection load='5c65' size='340' side='right' caption='Human glucose transporter complex with cholesterol derivative (PDB code [[5c65]])'> |
== Function == | == Function == | ||
GLUT3 is one of fourteen facilitative sugar transporters, which use the glucose diffusion gradient to move across various plasma membranes to display various specificities, kinetics and tissue expression profiles <ref name="three">Long, W., & Cheeseman, C. I. (2015). Structure of, and functional insight into the GLUT family of membrane transporters. Cell Health and Cytoskeleton, 7, 167-183. doi:10.2147/CHC.S60484</ref>. Glucose transporters are approximately 500 amino acids in length and part of a growing superfamily of integral membrane glycoproteins that have 12 transmembrane (TM) helices. The transmembrane regions presumably create channels through which glucose can move<ref name="four">Kipmen-Korgun, D., Bilmen-Sarikcioglu, S., Altunbas, H., Demir, R., & Korgun, E. T. (2009). Type-2 diabetes down-regulates glucose transporter proteins and genes of the human blood leukocytes.Scandinavian Journal of Clinical and Laboratory Investigation, 69(3), 350-358. | GLUT3 is one of fourteen facilitative sugar transporters, which use the glucose diffusion gradient to move across various plasma membranes to display various specificities, kinetics and tissue expression profiles <ref name="three">Long, W., & Cheeseman, C. I. (2015). Structure of, and functional insight into the GLUT family of membrane transporters. Cell Health and Cytoskeleton, 7, 167-183. doi:10.2147/CHC.S60484</ref>. Glucose transporters are approximately 500 amino acids in length and part of a growing superfamily of integral membrane glycoproteins that have 12 transmembrane (TM) helices. The transmembrane regions presumably create channels through which glucose can move<ref name="four">Kipmen-Korgun, D., Bilmen-Sarikcioglu, S., Altunbas, H., Demir, R., & Korgun, E. T. (2009). Type-2 diabetes down-regulates glucose transporter proteins and genes of the human blood leukocytes.Scandinavian Journal of Clinical and Laboratory Investigation, 69(3), 350-358. | ||
| Line 20: | Line 20: | ||
===Huntington’s Disease=== | ===Huntington’s Disease=== | ||
Huntington’s disease leads to decreased expression of GLUT3 in the plasma membrane. Increasing the expression of GLUT3 in a Huntington’s disease brain can delay the onset of the disease<ref name="ten">Vittori, A., Breda, C., Repici, M., Orth, M., Roos, R. A. C., Outeiro, T. F., . . . the REGISTRY investigators of the European Huntington's Disease Network. (2014). Copy-number variation of the neuronal glucose transporter gene SLC2A3 and age of onset in huntington's disease. Human Molecular Genetics, 23(12), 3129-3137. doi:10.1093/hmg/ddu022</ref>. Rab11 is a protein that is involved with the regulation of transporter trafficking. It helps in the regulation of glucose transporters particularly the GLUT3 transporter. Its regulation is impaired by Huntington’s disease, which leads to the decreased cell surface expression of GLUT3 in the brain. The exact mechanism of Huntington’s disease is still unknown to this day<ref name="eleven">McClory, H., Williams, D., & Sapp, E. (2014). Glucose transporter 3 is a rab11-dependent trafficking cargo and its transport to the cell surface is reduced in neurons of CAG140 Huntington’s disease mice. Acta Neuropathol Commun, 2, 1-9.</ref>. | Huntington’s disease leads to decreased expression of GLUT3 in the plasma membrane. Increasing the expression of GLUT3 in a Huntington’s disease brain can delay the onset of the disease<ref name="ten">Vittori, A., Breda, C., Repici, M., Orth, M., Roos, R. A. C., Outeiro, T. F., . . . the REGISTRY investigators of the European Huntington's Disease Network. (2014). Copy-number variation of the neuronal glucose transporter gene SLC2A3 and age of onset in huntington's disease. Human Molecular Genetics, 23(12), 3129-3137. doi:10.1093/hmg/ddu022</ref>. Rab11 is a protein that is involved with the regulation of transporter trafficking. It helps in the regulation of glucose transporters particularly the GLUT3 transporter. Its regulation is impaired by Huntington’s disease, which leads to the decreased cell surface expression of GLUT3 in the brain. The exact mechanism of Huntington’s disease is still unknown to this day<ref name="eleven">McClory, H., Williams, D., & Sapp, E. (2014). Glucose transporter 3 is a rab11-dependent trafficking cargo and its transport to the cell surface is reduced in neurons of CAG140 Huntington’s disease mice. Acta Neuropathol Commun, 2, 1-9.</ref>. | ||
| + | |||
| + | == 3D structure of sugar transporters == | ||
| + | |||
| + | See [[ABC transporter]] | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
Facilitated Glucose Transporter 3, Solute Carrier Family 2 (GLUT3/ SLC2A3) in Homo Sapiens
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Long, W., & Cheeseman, C. I. (2015). Structure of, and functional insight into the GLUT family of membrane transporters. Cell Health and Cytoskeleton, 7, 167-183. doi:10.2147/CHC.S60484
- ↑ 2.0 2.1 Kipmen-Korgun, D., Bilmen-Sarikcioglu, S., Altunbas, H., Demir, R., & Korgun, E. T. (2009). Type-2 diabetes down-regulates glucose transporter proteins and genes of the human blood leukocytes.Scandinavian Journal of Clinical and Laboratory Investigation, 69(3), 350-358. doi:10.1080/00365510802632163
- ↑ 3.0 3.1 Simpson,I. A., Dwyer, D., Malide, D., Moley, K. H., Travis, A., & Vannucci, S. J. (2008). The facilitative glucose transporter GLUT3: 20 years of distinction. American Journal of Physiology - Endocrinology and Metabolism, 295(2), E242-E253. doi:10.1152/ajpendo.90388.2008
- ↑ Maher, F., Vannucci, S. J., & Simpson, I. A. (1994). Glucose transporter proteins in brain. FASEB Journal, 8(13), 1003-1011.
- ↑ Xu, J., Lu, C., Wang, J., Zhang, R., Qian, X., & Zhu, H. (2015). Regulation of human trophoblast GLUT3 glucose transporter by mammalian target of rapamycin signaling. International Journal of Molecular Sciences, 16(6), 13815-13828. doi:10.3390/ijms160613815
- ↑ 6.0 6.1 Liu, Y., Liu, F., Iqbal, K., Grundke-Iqbal, I., & Gong, C. -. (2008). Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in alzheimer disease. FEBS Letters, 582(2), 359-364. doi:10.1016/j.febslet.2007.12.035
- ↑ 7.0 7.1 http://www.ebi.ac.uk/pdbe/entry/pdb/5c65/
- ↑ http://oca.weizmann.ac.il/oca-bin/ocaids?id=5c65
- ↑ 9.0 9.1 9.2 9.3 Deng, D., Sun, P., Yan, C., Ke, M., Jiang, X., Xiong, L., . . . Yan, N. (2015). Molecular basis of ligand recognition and transport by glucose transporters. Nature, 526(7573), 391-396. doi:10.1038/nature14655
- ↑ http://www.rcsb.org/pdb/explore.do?structureId=5C65
- ↑ http://www.ebi.ac.uk/pdbe/entry/pdb/5c65/bound/37X
- ↑ http://www.ebi.ac.uk/pdbe/entry/pdb/5c65/bound/Y01
- ↑ 13.0 13.1 Naftalin RJ, Holman GD. Transport of sugars in human red cells. In: Ellory JC, Lew V, editors. \ Membrane Transport in Red Cells. New York, NY, USA: Academic Press; 1977.
- ↑ 14.0 14.1 Carruthers, A., DeZutter, J., Ganguly, A., & Devaskar, S. U. (2009). Will the original glucose transporter isoform please stand up! American Journal of Physiology - Endocrinology and Metabolism, 297(4), E836-E848. doi:10.1152/ajpendo.00496.2009
- ↑ Jardetzky, O. (1966). Simple allosteric model for membrane pumps [27]. Nature, 211(5052), 969-970. doi:10.1038/211969a0
- ↑ Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–615.
- ↑ Caulfield MJ, Munroe PB, O’Neill D, et al. SLC2A9 is a high-capacity urate transporter in humans. PLoS Med. 2008;5:1509–1523.
- ↑ Vollers, S. S., & Carruthers, A. (2012). Sequence determinants of GLUT1-mediated accelerated-exchange transport: Analysis by homology-scanning mutagenesis. Journal of Biological Chemistry, 287(51), 42533-42544.doi:10.1074/jbc.M112.369587
- ↑ Vittori, A., Breda, C., Repici, M., Orth, M., Roos, R. A. C., Outeiro, T. F., . . . the REGISTRY investigators of the European Huntington's Disease Network. (2014). Copy-number variation of the neuronal glucose transporter gene SLC2A3 and age of onset in huntington's disease. Human Molecular Genetics, 23(12), 3129-3137. doi:10.1093/hmg/ddu022
- ↑ McClory, H., Williams, D., & Sapp, E. (2014). Glucose transporter 3 is a rab11-dependent trafficking cargo and its transport to the cell surface is reduced in neurons of CAG140 Huntington’s disease mice. Acta Neuropathol Commun, 2, 1-9.
Proteopedia Page Contributors and Editors (what is this?)
Kevin Keaveney, Michal Harel, Javier Blanco, Matthew J Lowry, Lainey Scarvey, Samantha Chinn, Miranda Moore