Sandbox Reserved 1121
From Proteopedia
| (118 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | + | <Structure load='1B09' size='350' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /> | |
| - | + | {{Human C-reactive protein complexed with phosphocholine}} | |
| - | + | ||
| - | + | Human C-Reactive Protein (CRP) is an acute phase protein belonging to the highly conserved pentraxin protein family, such as its homologue, the Serum Amyloid P component (SAP)<ref name="Thompson">PMID: 10368284</ref>. CRP was named this way because it was found that the protein could precipitate the "C" polysaccharide derived from ''Streptococcus pneumoniae'' cell wall<ref name="Black">PMID: 15337754</ref><ref name="kumar">PMID: 21541851</ref>. | |
| - | + | ||
| + | Although CRP is a normal serum protein (healthy humans have a CRP rate which is generally about 1 μg/mL<ref name="kumar"/>), its circulating concentration rises rapidly and extensively (as much as 1000-fold or more<ref name="Black"/>) in a cytokine-mediated way in response to an infection, an inflammation or a tissue injury. In this way, serum CRP rate is empirically dosed to detect many human diseases<ref name="Thompson"/>. CRP can be defined as a target for the development of cardioprotection and neuroprotection<ref name="kumar"/>. CRP is secreted by the liver into the blood circulation<ref name = "Alex"/> during inflammatory states: the acute phase response, in which the synthesis of a lot of plasma proteins increases<ref name="Black"/>. | ||
| + | |||
== Structure == | == Structure == | ||
| + | === CRP structure === | ||
| + | |||
| + | The crystal structure of CRP was determined by using SAP as a research model<ref name="Thompson"/>. The structure of CRP has been obtained by X-ray crystallography at 3 Å resolution. Like SAP, the protein consists of five protomers, uncovalently bonded and nonglycosylated that are arranged symmetrically around a central pore<ref name="Volanakis">PMID: 11532280</ref>. Each subunit is a 25 kDa protein consisting of 224 residues<ref name="uniprot">[http://www.uniprot.org/uniprot/P02741 UniProtKB - P02741 (CRP_HUMAN)]</ref>. The diameter of the CRP pentamer is 102 Å, the inner pore diameter is 30 Å and the diameter of a subunit is 36 Å<ref name="agrawal">PMID: 19799114 </ref><ref name="Volanakis"/>. Ser53, His95, Cys97, Asp112, Gly113, Gly136, Gly154, Val165, Leu166, Ile171, and Gly196 are the highliest conserved residues in the primary sequence of CRP<ref name="kumar"/>. | ||
| + | |||
| + | Each subunit consists of two antiparallel <scene name='71/719862/Sheet/1'>β-sheets</scene><ref name="uniprot"/> with a flattened jellyroll topology<ref name="Volanakis"/> and a long <scene name='71/719862/Helix/1'>α-helix</scene> (residues 168-176) that lies folded against the β-sheets. The predominant structure is β-sheet<ref>PMID: 1382589</ref> but short helical regions can be noticed for the residues 43 and 185<ref name="kumar"/>. The carboxyl terminal end of the helix along with the loop 177-182 forms one of the two sides of a cleft that extends from the centre of the protomer to its edge at the central pore of the pentamer<ref name="Volanakis"/>. The other side of the cleft is formed of parts of the amino and carboxyl termini of the protomer<ref name="Volanakis"/>. This furrow is 24 Å long, 7.5 Å deep and 12.4 Å wide. The side walls are constructed from Ser5, Arg6, Gln203, Pro206, Trp187, Arg188, Asn160, Gly177, Leu176, Tyr175, His95 and Asp112<ref name="Thompson"/>. This cleft is involved in C1q binding and perhaps also with the neutrophils Fc Receptor<ref name="duclos">PMID: 15531769 </ref><ref name="Volanakis"/>.The outer part of the furrow is positively charged but the inner part terminates halfway through the pentamer pore at residue Asp112, providing a ring of negative charges lining the pore<ref name="Thompson"/>. Asp112 seems to be an important residue for recognition of Cq1 by CRP<ref name="Thompson"/> because it is considered, with Tyr175, to be C1q contact residues. Glu88 seems to influence the conformational change of C1q necessary for complement activation, while Asn158 and His38 may contribute to the correct geometry of the binding site<ref name="Volanakis"/>. | ||
| + | |||
| + | The cleft that binds C1q and Fc-Receptor is on the face A of a CRP protomer. On the other face of the protomer (face B), two calcium ions are bound 4 Å apart by protein sidechains coming from loops collected at the concave face (face A). The calcium ion and the loop on the face B form the phosphocholine (PC)-binding site<ref name="Thompson"/>. Each subunit in CRP is rotated by 22° towards the fivefold axis so that the helices of face A are 5 Å closer to the axis and the calcium sites on face B move away by an equivalent amount<ref name="Volanakis"/><ref name="Thompson"/>. | ||
| + | Thus, the CRP pentamer has two faces: a face that exibits the five PC-binding sites and a face involved in the binding of C1q and possibly FcR<ref name="Volanakis"/>. | ||
| + | |||
| + | The five protomers are related one with another by three salt bridges that mainly involve the 115-123 loop of one protomer and the 40-42 and 197-202 regions of the other protomer<ref name="Volanakis"/>. | ||
| + | |||
| + | === Ca<sup>2+</sup>-binding site === | ||
| + | [[Image:Loop Ca2+.PNG | thumb | Loop involved in Ca<sup>2+</sup>-binding site (left), Ca<sup>2+</sup>-depleted structure (right)<ref name="PyMol">The PyMOL Molecular Graphics System, Version 1.8 Schrödinger, LLC.</ref>]] | ||
| + | CRP is a calcium dependent structure. The face B binds two Ca<sup>2+</sup> ions per subunit. The ligands are bound asymmetrically in both Ca<sup>2+</sup>-binding sites. The residues of the site 1 include Asp60, Asn61, Glu138, Asp140, the carbonyl oxygen of the residue 139 and one oxygen of Asp60. The site 1 provides a total of five ligands for the Ca<sup>2+</sup> ion. The site 2 residues include Gln138, Asp140, Gln150 and non-coordinating Glu147. The site 2 provides four ligands for the Ca<sup>2+</sup> ion. The two Ca<sup>2+</sup>-binding sites are of equal affinity for Ca<sup>2+</sup> and are only differentiated by the carbonyl oxygen ligand. In presence of Ca<sup>2+</sup>, the two Ca<sup>2+</sup>-binding sites are overlapping in a loop. When the Ca<sup>2+</sup> sites are free, the residues 140 to 150 form a loop away from the molecule<ref name="Thompson"/>. This releases the proteolysis site<ref name="agrawal"/>, which induces the cleavage of CRP between Asn145 and Phe146 by nagarase protease and between Phe146 and Glu147 by pronase<ref name="ramadan">PMID: 12037301</ref>. Therefore Ca<sup>2+</sup> protects CRP form proteolytic cleavage and from degradation<ref name="agrawal"/>. | ||
| + | |||
| + | === Phosphocholine-binding site === | ||
| + | [[Image:Phosphocholine.PNG | thumb | PC and Ca<sup>2+</sup>-binding site<ref name="PyMol"/>]] | ||
| + | Phosphocholine (PC) is a lipoprotein found in cell membranes and in plasma<ref name="Thompson"/>. The PC-binding site is a hydrophobic pocket constituted by the residues Leu64, Phe66, Thr76, Glu81 and the two Ca<sup>2+</sup><ref name="agrawal"/><ref name="Volanakis"/>. These residues are very important because if one of them is mutated into another, the avidity for PC is significantly reduced<ref name="Volanakis"/>. | ||
| + | |||
| + | Two of the oxygens of the phosphate group directly co-ordinate with the two Ca<sup>2+</sup> bound on the CRP<ref name="Volanakis"/>, leaving the third oxygen pointing away from the binding sites<ref name="Thompson"/>. The choline group stays within the hydrophobic pocket. | ||
| + | The three choline methyl groups make hydrophobic interactions with the exposed face of Phe66, while the positively charged quaternary choline nitrogen interacts with Glu81 which is located on the other side of the pocket<ref name="Volanakis"/>. | ||
| + | |||
| + | The affinity of CRP for PC increases when the concentration of PC does so. A surface containing a high density of PC, such as C-polycaccharide, is therefore propitious to the CRP-binding<ref name="duclos"/>. CRP can also bind chromatin, histones, small nuclear ribonucleoproteins, nuclear envelope proteins and nucleosomes in a Ca<sup>2+</sup>-dependent manner<ref name="agrawal"/>. | ||
| + | |||
| + | |||
| + | == Function == | ||
| + | CRP is involved in the first line of innate host defense. Its main function is to clear bacterial pathogens and apoptotic and necrotic cells. In fact, CRP binds to PC located on the surface of pathogenic bacteria that infected the organism. The resulting immune response is the phagocytosis of PC-expressing bacteria<ref name="agrawal"/>. PC is present in teichoic acids, capsular carbohydrates, and lipopolysaccharides of bacteria and other micro-organisms. There are many micro-organisms that synthetize PC, such as ''Streptococcus pneumoniae'', ''Haemophilus influenzae'', ''Pseudomonas aeruginosa'', ''Neisseria meningitides'' and ''Neisseria gonorrhoeae'', ''Proteus morganii and Aspergillus fumigatus''<ref name="Volanakis"/>. | ||
| + | |||
| + | Because the five PC-binding sites are on the face B of the pentamer, CRP binds with high probability to ligand-covered targets such as bacteria or other biological membranes. On its other face, CRP is able to recognize C1q or Fc receptors which have an inflammatory effect<ref name = "Alex">PMID: 12616974</ref>. Macrophages synthetize membrane C1q and Fc-receptors that will bind to the face A of CRP. C1q and Fc are opsonins, which means that they are molecules that enhance phagocytosis by marking an antigen for an immune response. Therefore, they mark the targeted cell as an antigen. The macrophages have C1q and FC-binding site, they will therefore detect the marked cell and clear it<ref name = "Terheyden">PMID: 16023736</ref>. | ||
| - | == | + | == Biomedical consideration == |
| - | + | ||
| - | = | + | It is known that CRP rate increases rapidly and remarkably during inflammatory states. However, minor CRP elevation has been related with future major cardiovascular events. Indeed, studies have shown that a slightly elevated CRP plasma level (between 3 and 10 µg/mL) is associated with a risk of developing cardiovascular disease, metabolic syndrome and colon cancer<ref name="Black"/>. |
| - | = | + | Nevertheless, minor increase of CRP level is not necessarly associated with inflammation but could be linked to several genetic polymorphisms of CRP and other gens, ethnicity, various dietary patterns and obesity<ref name="Black"/>. |
| - | + | Futhermore, CRP might play a role in the pathogenesis of atherosclerosis, but the exact role of CRP in atherosclerosis is not known yet. It was found that CRP binds the phosphocholine of oxidized low density lipoproteins, up-regulates the expression of adhesion molecules in endothelial cells, increases low density lipoprotein uptake into macrophages, inhibits endothelial nitric-oxide synthase expression in aortic endothelial cells and increases plasminogen activator inhibitor-1 expression and activity<ref name="Black"/>. Transgenic mice deficient in apolipoprotein E and expressing high levels of CRP display a modest acceleration in aortic atherosclerosis<ref name="Black"/>. | |
| - | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
|
Template:Human C-reactive protein complexed with phosphocholine
Human C-Reactive Protein (CRP) is an acute phase protein belonging to the highly conserved pentraxin protein family, such as its homologue, the Serum Amyloid P component (SAP)[1]. CRP was named this way because it was found that the protein could precipitate the "C" polysaccharide derived from Streptococcus pneumoniae cell wall[2][3].
Although CRP is a normal serum protein (healthy humans have a CRP rate which is generally about 1 μg/mL[3]), its circulating concentration rises rapidly and extensively (as much as 1000-fold or more[2]) in a cytokine-mediated way in response to an infection, an inflammation or a tissue injury. In this way, serum CRP rate is empirically dosed to detect many human diseases[1]. CRP can be defined as a target for the development of cardioprotection and neuroprotection[3]. CRP is secreted by the liver into the blood circulation[4] during inflammatory states: the acute phase response, in which the synthesis of a lot of plasma proteins increases[2].
Contents |
Structure
CRP structure
The crystal structure of CRP was determined by using SAP as a research model[1]. The structure of CRP has been obtained by X-ray crystallography at 3 Å resolution. Like SAP, the protein consists of five protomers, uncovalently bonded and nonglycosylated that are arranged symmetrically around a central pore[5]. Each subunit is a 25 kDa protein consisting of 224 residues[6]. The diameter of the CRP pentamer is 102 Å, the inner pore diameter is 30 Å and the diameter of a subunit is 36 Å[7][5]. Ser53, His95, Cys97, Asp112, Gly113, Gly136, Gly154, Val165, Leu166, Ile171, and Gly196 are the highliest conserved residues in the primary sequence of CRP[3].
Each subunit consists of two antiparallel [6] with a flattened jellyroll topology[5] and a long (residues 168-176) that lies folded against the β-sheets. The predominant structure is β-sheet[8] but short helical regions can be noticed for the residues 43 and 185[3]. The carboxyl terminal end of the helix along with the loop 177-182 forms one of the two sides of a cleft that extends from the centre of the protomer to its edge at the central pore of the pentamer[5]. The other side of the cleft is formed of parts of the amino and carboxyl termini of the protomer[5]. This furrow is 24 Å long, 7.5 Å deep and 12.4 Å wide. The side walls are constructed from Ser5, Arg6, Gln203, Pro206, Trp187, Arg188, Asn160, Gly177, Leu176, Tyr175, His95 and Asp112[1]. This cleft is involved in C1q binding and perhaps also with the neutrophils Fc Receptor[9][5].The outer part of the furrow is positively charged but the inner part terminates halfway through the pentamer pore at residue Asp112, providing a ring of negative charges lining the pore[1]. Asp112 seems to be an important residue for recognition of Cq1 by CRP[1] because it is considered, with Tyr175, to be C1q contact residues. Glu88 seems to influence the conformational change of C1q necessary for complement activation, while Asn158 and His38 may contribute to the correct geometry of the binding site[5].
The cleft that binds C1q and Fc-Receptor is on the face A of a CRP protomer. On the other face of the protomer (face B), two calcium ions are bound 4 Å apart by protein sidechains coming from loops collected at the concave face (face A). The calcium ion and the loop on the face B form the phosphocholine (PC)-binding site[1]. Each subunit in CRP is rotated by 22° towards the fivefold axis so that the helices of face A are 5 Å closer to the axis and the calcium sites on face B move away by an equivalent amount[5][1]. Thus, the CRP pentamer has two faces: a face that exibits the five PC-binding sites and a face involved in the binding of C1q and possibly FcR[5].
The five protomers are related one with another by three salt bridges that mainly involve the 115-123 loop of one protomer and the 40-42 and 197-202 regions of the other protomer[5].
Ca2+-binding site
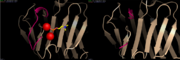
CRP is a calcium dependent structure. The face B binds two Ca2+ ions per subunit. The ligands are bound asymmetrically in both Ca2+-binding sites. The residues of the site 1 include Asp60, Asn61, Glu138, Asp140, the carbonyl oxygen of the residue 139 and one oxygen of Asp60. The site 1 provides a total of five ligands for the Ca2+ ion. The site 2 residues include Gln138, Asp140, Gln150 and non-coordinating Glu147. The site 2 provides four ligands for the Ca2+ ion. The two Ca2+-binding sites are of equal affinity for Ca2+ and are only differentiated by the carbonyl oxygen ligand. In presence of Ca2+, the two Ca2+-binding sites are overlapping in a loop. When the Ca2+ sites are free, the residues 140 to 150 form a loop away from the molecule[1]. This releases the proteolysis site[7], which induces the cleavage of CRP between Asn145 and Phe146 by nagarase protease and between Phe146 and Glu147 by pronase[11]. Therefore Ca2+ protects CRP form proteolytic cleavage and from degradation[7].
Phosphocholine-binding site
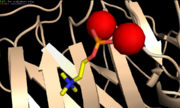
Phosphocholine (PC) is a lipoprotein found in cell membranes and in plasma[1]. The PC-binding site is a hydrophobic pocket constituted by the residues Leu64, Phe66, Thr76, Glu81 and the two Ca2+[7][5]. These residues are very important because if one of them is mutated into another, the avidity for PC is significantly reduced[5].
Two of the oxygens of the phosphate group directly co-ordinate with the two Ca2+ bound on the CRP[5], leaving the third oxygen pointing away from the binding sites[1]. The choline group stays within the hydrophobic pocket. The three choline methyl groups make hydrophobic interactions with the exposed face of Phe66, while the positively charged quaternary choline nitrogen interacts with Glu81 which is located on the other side of the pocket[5].
The affinity of CRP for PC increases when the concentration of PC does so. A surface containing a high density of PC, such as C-polycaccharide, is therefore propitious to the CRP-binding[9]. CRP can also bind chromatin, histones, small nuclear ribonucleoproteins, nuclear envelope proteins and nucleosomes in a Ca2+-dependent manner[7].
Function
CRP is involved in the first line of innate host defense. Its main function is to clear bacterial pathogens and apoptotic and necrotic cells. In fact, CRP binds to PC located on the surface of pathogenic bacteria that infected the organism. The resulting immune response is the phagocytosis of PC-expressing bacteria[7]. PC is present in teichoic acids, capsular carbohydrates, and lipopolysaccharides of bacteria and other micro-organisms. There are many micro-organisms that synthetize PC, such as Streptococcus pneumoniae, Haemophilus influenzae, Pseudomonas aeruginosa, Neisseria meningitides and Neisseria gonorrhoeae, Proteus morganii and Aspergillus fumigatus[5].
Because the five PC-binding sites are on the face B of the pentamer, CRP binds with high probability to ligand-covered targets such as bacteria or other biological membranes. On its other face, CRP is able to recognize C1q or Fc receptors which have an inflammatory effect[4]. Macrophages synthetize membrane C1q and Fc-receptors that will bind to the face A of CRP. C1q and Fc are opsonins, which means that they are molecules that enhance phagocytosis by marking an antigen for an immune response. Therefore, they mark the targeted cell as an antigen. The macrophages have C1q and FC-binding site, they will therefore detect the marked cell and clear it[12].
Biomedical consideration
It is known that CRP rate increases rapidly and remarkably during inflammatory states. However, minor CRP elevation has been related with future major cardiovascular events. Indeed, studies have shown that a slightly elevated CRP plasma level (between 3 and 10 µg/mL) is associated with a risk of developing cardiovascular disease, metabolic syndrome and colon cancer[2].
Nevertheless, minor increase of CRP level is not necessarly associated with inflammation but could be linked to several genetic polymorphisms of CRP and other gens, ethnicity, various dietary patterns and obesity[2].
Futhermore, CRP might play a role in the pathogenesis of atherosclerosis, but the exact role of CRP in atherosclerosis is not known yet. It was found that CRP binds the phosphocholine of oxidized low density lipoproteins, up-regulates the expression of adhesion molecules in endothelial cells, increases low density lipoprotein uptake into macrophages, inhibits endothelial nitric-oxide synthase expression in aortic endothelial cells and increases plasminogen activator inhibitor-1 expression and activity[2]. Transgenic mice deficient in apolipoprotein E and expressing high levels of CRP display a modest acceleration in aortic atherosclerosis[2].
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 Thompson D, Pepys MB, Wood SP. The physiological structure of human C-reactive protein and its complex with phosphocholine. Structure. 1999 Feb 15;7(2):169-77. PMID:10368284
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Black S, Kushner I, Samols D. C-reactive Protein. J Biol Chem. 2004 Nov 19;279(47):48487-90. Epub 2004 Aug 26. PMID:15337754 doi:http://dx.doi.org/10.1074/jbc.R400025200
- ↑ 3.0 3.1 3.2 3.3 3.4 Kumar SV, Ravunny RK, Chakraborty C. Conserved domains, conserved residues, and surface cavities of C-reactive protein (CRP). Appl Biochem Biotechnol. 2011 Sep;165(2):497-505. doi: 10.1007/s12010-011-9270-7., Epub 2011 May 4. PMID:21541851 doi:http://dx.doi.org/10.1007/s12010-011-9270-7
- ↑ 4.0 4.1 Szalai AJ. The biological functions of C-reactive protein. Vascul Pharmacol. 2002 Aug;39(3):105-7. PMID:12616974
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 Volanakis JE. Human C-reactive protein: expression, structure, and function. Mol Immunol. 2001 Aug;38(2-3):189-97. PMID:11532280
- ↑ 6.0 6.1 UniProtKB - P02741 (CRP_HUMAN)
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 Agrawal A, Singh PP, Bottazzi B, Garlanda C, Mantovani A. Pattern recognition by pentraxins. Adv Exp Med Biol. 2009;653:98-116. PMID:19799114
- ↑ Dong A, Caughey B, Caughey WS, Bhat KS, Coe JE. Secondary structure of the pentraxin female protein in water determined by infrared spectroscopy: effects of calcium and phosphorylcholine. Biochemistry. 1992 Oct 6;31(39):9364-70. PMID:1382589
- ↑ 9.0 9.1 Du Clos TW, Mold C. C-reactive protein: an activator of innate immunity and a modulator of adaptive immunity. Immunol Res. 2004;30(3):261-77. PMID:15531769 doi:http://dx.doi.org/10.1385/IR:30:3:261
- ↑ 10.0 10.1 The PyMOL Molecular Graphics System, Version 1.8 Schrödinger, LLC.
- ↑ Ramadan MA, Shrive AK, Holden D, Myles DA, Volanakis JE, DeLucas LJ, Greenhough TJ. The three-dimensional structure of calcium-depleted human C-reactive protein from perfectly twinned crystals. Acta Crystallogr D Biol Crystallogr. 2002 Jun;58(Pt 6 Pt 2):992-1001. Epub, 2002 May 29. PMID:12037301
- ↑ Terheyden P, Loos M, Storkel S, Kaul M. Human macrophages simultaneously express membrane-C1q and Fc-receptors for IgG. Immunol Lett. 2005 Nov 15;101(2):202-9. PMID:16023736 doi:http://dx.doi.org/10.1016/j.imlet.2005.06.002
