Sandbox Reserved 1136
From Proteopedia
(Difference between revisions)
| (31 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | {{Sandbox_Reserved_ESBS_2015}} | + | {{Sandbox_Reserved_ESBS_2015}} |
| - | ==Structure and Functional aspects of Sucrose Synthase from ''Arabidopsis thaliana''== | + | ==Structure and Functional aspects of Sucrose Synthase 1 from ''Arabidopsis thaliana''== |
| - | + | Sucrose Synthase 1 (EC:2.4.1.13), also known as the sucrose-UDP glucolsyltransferase 1, is a reversible enzyme allowing the synthesis or the degradation of sucrose in ''Arabidopsis thaliana''. It is a 360 kDa tetramer and belongs to the Glycosyltransferase subfamily 4 (GT4). | |
| - | Sucrose Synthase 1 (EC:2.4.1.13), also known as the | + | |
| + | <StructureSection scene='71/719877/Susy/7' size='340' side='right' caption='X-ray crystal structures of AtSus1, as a complex with UDP-glucose at 2.8-Å resolution and as a complex with UDP and fructose at 2.85-Å resolution'> | ||
== Function == | == Function == | ||
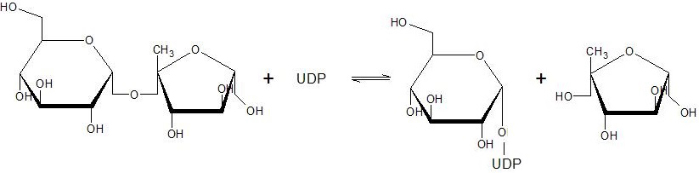
| - | The Sucrose Synthase is able to catalyse the following reaction: | + | The Sucrose Synthase is able to catalyse the following reaction in both directions: |
| + | [[Image:Susy_reaction.jpg]] | ||
| - | '''sucrose | + | This reaction is a nucleophilic substitution involving a glycosyl intermediate. The glucose is transfered between UDP (donor) and fructose (acceptor). |
| - | + | ||
| + | This enzyme has a fundamental role in the sucrose flow regulation since it is able to produce sucrose (used for transportation and stockage) and both ADP-glucose and UDP-glucose. It is one of the only four proteins able to synthesize or cleave the sucrose, and the only one able to catalyze it in both direction <ref>Salerno GL, Curatti L | ||
| + | Origin of sucrose metabolism in higher plants: when, how and why? | ||
| + | Trends Plant Sci. 2003 Feb | ||
| + | </ref>, thereby Sucrose synthase is implied in many pathways including cell wall, starch and glycoprotein synthesis <ref>Baroja-Fernández, E., Muñoz, F.J., Saikusa, T., Rodríguez-López, M., Akazawa, T. and Pozueta-Romero, J. Sucrose synthase catalyzes the de novo production of ADP-glucose linked to starch biosynthesis in heterotrophic tissues of plants. Plant Cell Physiol.</ref>. | ||
| + | Sucrose synthase catalyses the cleavage reaction at pH around 7 and the synthesis reaction at pH above 9. Cytoplasmic pH is around 6,5-7, it suggests that SUS works ''in vivo'' only to cleave sucrose, which explain why SUS1 is mostly in sink organs. Therefore, it is a linker between all the pathways that use or synthesize sucrose. SUS is involved in biomass production, nitrogen fixation, fruit and seed maturation and can response to stress (cold, anaerobic conditions, drought or gene induction). | ||
| + | However, the binding to target mechanism is unknown at the molecular level. | ||
| + | |||
| + | In a mutant ''Sus1'' (SUS1 gene repression), we observe a decrease in amidon and lipids concentration, a misbalance between hexose and sucrose, and a perturbation of the organic acids synthesis (citrate and malate), during seed maturation | ||
| + | <ref>11 Juan Gabriel Angeles N_U ~Nez. Study of sucrose synthase in arabdopsis seed : lacalization, | ||
| + | regulation and function. Sciences of the U.</ref>. | ||
| - | It has a fundamental role in the Sucrose flow regulation since it is able to produce both Sucrose (used for transport) and Glucose. It is one of the only four proteins able to synthesize or cleave the Sucrose. Thereby Sucrose synthase is implied in many pathways including cell wall, starch and glycoprotein synthesis. | ||
== Structural highlights == | == Structural highlights == | ||
| - | + | Sucrose synthase is normally a homotetrameric enzyme, but it can also exist in a dimer form | |
| + | <ref>Sucrose synthase oligomerization and F-actin association are regulated by sucrose concentration and phosphorylation. Duncan KA, Huber SC. Plant Cell Physiol. 2007 Nov; 48(11):1612-23. | ||
| + | </ref>. | ||
| + | There are 6 SUS isoforms in ''Arabidopsis thaliana'' and all of them are structurally similar to sucrose phosphate synthases and glycogen synthases. <ref>Salerno GL, Curatti L. Origin of sucrose metabolism in higher plants: when, how and why? Trends Plant Sci. 2003 Feb</ref>. | ||
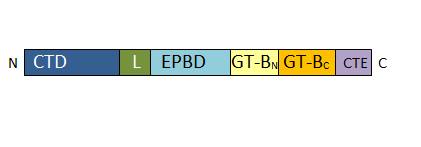
| + | Each monomer is a chain of 808 residues which possesses four specific domains: | ||
| + | • 1-127: N-Terminal regulatory domain involved in targeting (Cellular Targeting Domain) <ref>Determination of structural requirements and probable regulatory effectors for membrane association of maize Sucrose Synthase 1. Hardin SC, Duncan KA, Huber SC. Plant Physiol. 2006</ref>. On this sequence, two serines can be phosphorylated, which enable a control of enzyme location <ref>Phosphorylation of Sucrose Synthase at serine 170: occurrence and possible role as a signal for proteolysis. Hardin SC, Tang GQ, Scholz A, Holtgraewe D, Winter H, Huber SC. Plant J. 2003</ref>. | ||
| + | |||
| + | • 157-276: EPBD: ENOD40 peptide-binding domain. This domain has a role in the regulation of the enzyme. It is able to bind a potassium ion. | ||
| + | |||
| + | • 277-776 : GT-B glycosyltransferase domain. It contains the catalytic site and presents a characteristic Rossman-folding | ||
| + | <ref>Glycosyltransferases: structures, functions, and mechanisms. Lairson LL, Henrissat B, Davies GJ, Withers SG. Annu Rev Biochem. 2008 | ||
| + | </ref>. It is divided in two parts, the GT-BN and the GT-BC. | ||
| + | |||
| + | • 776-808 : C-terminal extension. The length of this domain is variable depending of the SUS isoform. | ||
| + | |||
| + | [[Image:Monomer structure.jpg]] | ||
| + | ''Primary structure of Atsus1 monomer. CTD : for Cellular Targeting Domain (residues 0-127); L for Linker (residues 128-156); EPBD: for ENOD40 Peptide-Binding Domain (residues 157-276); GT-B glycosyltransferase : Gt-BN (residues 277-526) and Gt-BC (residues 527-754); CTE for C-Terminal Extension (residues 776-808)'' | ||
| + | |||
| + | |||
| + | The SUS1 tetramer is flat, with two types of hydrophobic subunit interfaces, the A:B and A:D interfaces. The A:D interface is an interaction between the C-terminal extension and the linker, whereas the A:B interface is created by the interaction of adjacent EPBD domains. | ||
| + | |||
| + | The active site of SUS1 is able to bind both fructose and UDP-glucose. UDP-glucose is mainly bound by the GT-BC domain, whereas the fructose in β-furanose form is bound within a pocket in the GT-BN domain. | ||
| + | |||
| + | The GT-B domain is highly conserved in other isoforms and in the Sucrose-Phosphate Synthase. This conservation reinforce the evolutionary relationship of those enzymes. Furthermore, this domain is also conserved in other species. | ||
| + | |||
| + | == Regulation == | ||
| + | Sucrose Synthase regulation is not well known, but here are some of the supposed mechanisms. | ||
| + | |||
| + | • ENOD40-A is a small hormon-like peptide able to specifically thiolates the Cys-266 of AtSUS1. It also inhibits the phosphorylation of Ser-167, which is within the A:B interface : in this way, in the native tetramer, Ser-167 is inaccessible to phosphorylation, but would be accessible if the tetramer dissociates into dimers upon breaking of the A:B interface (because this interface is less hydrophobic than the A:D interface). So the dissociation into dimers would then allow its phosphorylation and increase the potential for ubiquitinylation and thereby turnover. | ||
| + | |||
| + | • In Arabidopsis, SUS1 gene is probably regulated both by sucrose and D-glucose. | ||
| + | |||
| + | • Conformational changes of the EPBD may change the active site activity through distortions of one of the α helix. | ||
| + | |||
| + | • The CTD can possibly move as a rigid body and interact with F-actin, where the groove and EPBD can be in contact with actin fiber. It may create a pathway for actin binding, and modulation of the Sucrose Synthase activity. | ||
| + | |||
| + | • In other organisms, it has been shown that the Sucrose Synthase is active into its dimer form, it is supposed to be the same phenomenon in ''Arabidopsis thaliana'', though it has not been prouved. | ||
</StructureSection> | </StructureSection> | ||
| Line 22: | Line 68: | ||
== References == | == References == | ||
| + | • 3D structure and informations mainly extracted from: The Structure of Sucrose Synthase-1 from Arabidopsis thaliana and Its Functional Implications. Zheng, Y., Anderson, S., Zhang, Y., Garavito, R.M. (2011) J.Biol.Chem. 286: 36108-36118. | ||
| + | |||
• UniProt entry: P49040 | • UniProt entry: P49040 | ||
Current revision
| This Sandbox is Reserved from 15/12/2015, through 15/06/2016 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1120 through Sandbox Reserved 1159. |
To get started:
More help: Help:Editing |
Structure and Functional aspects of Sucrose Synthase 1 from Arabidopsis thaliana
Sucrose Synthase 1 (EC:2.4.1.13), also known as the sucrose-UDP glucolsyltransferase 1, is a reversible enzyme allowing the synthesis or the degradation of sucrose in Arabidopsis thaliana. It is a 360 kDa tetramer and belongs to the Glycosyltransferase subfamily 4 (GT4).
| |||||||||||
References
• 3D structure and informations mainly extracted from: The Structure of Sucrose Synthase-1 from Arabidopsis thaliana and Its Functional Implications. Zheng, Y., Anderson, S., Zhang, Y., Garavito, R.M. (2011) J.Biol.Chem. 286: 36108-36118.
• UniProt entry: P49040
• Brenda entry : 2.4.1.13
- ↑ Salerno GL, Curatti L Origin of sucrose metabolism in higher plants: when, how and why? Trends Plant Sci. 2003 Feb
- ↑ Baroja-Fernández, E., Muñoz, F.J., Saikusa, T., Rodríguez-López, M., Akazawa, T. and Pozueta-Romero, J. Sucrose synthase catalyzes the de novo production of ADP-glucose linked to starch biosynthesis in heterotrophic tissues of plants. Plant Cell Physiol.
- ↑ 11 Juan Gabriel Angeles N_U ~Nez. Study of sucrose synthase in arabdopsis seed : lacalization, regulation and function. Sciences of the U.
- ↑ Sucrose synthase oligomerization and F-actin association are regulated by sucrose concentration and phosphorylation. Duncan KA, Huber SC. Plant Cell Physiol. 2007 Nov; 48(11):1612-23.
- ↑ Salerno GL, Curatti L. Origin of sucrose metabolism in higher plants: when, how and why? Trends Plant Sci. 2003 Feb
- ↑ Determination of structural requirements and probable regulatory effectors for membrane association of maize Sucrose Synthase 1. Hardin SC, Duncan KA, Huber SC. Plant Physiol. 2006
- ↑ Phosphorylation of Sucrose Synthase at serine 170: occurrence and possible role as a signal for proteolysis. Hardin SC, Tang GQ, Scholz A, Holtgraewe D, Winter H, Huber SC. Plant J. 2003
- ↑ Glycosyltransferases: structures, functions, and mechanisms. Lairson LL, Henrissat B, Davies GJ, Withers SG. Annu Rev Biochem. 2008