This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 1126
From Proteopedia
(Difference between revisions)
| (5 intermediate revisions not shown.) | |||
| Line 35: | Line 35: | ||
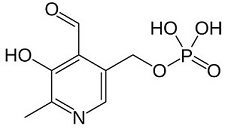
*<scene name='71/719867/Scene_4/1'>Pyridoxal phosphate (PLP)</scene> <ref>http://www.ebi.ac.uk/interpro/entry/IPR004625</ref> <ref>https://pubchem.ncbi.nlm.nih.gov/compound/pyridoxal_phosphate#section=Top</ref> | *<scene name='71/719867/Scene_4/1'>Pyridoxal phosphate (PLP)</scene> <ref>http://www.ebi.ac.uk/interpro/entry/IPR004625</ref> <ref>https://pubchem.ncbi.nlm.nih.gov/compound/pyridoxal_phosphate#section=Top</ref> | ||
| + | |||
<center>[[Image:Pyridoxal phosphate.jpg]] © Wikipedia</center> <br/> | <center>[[Image:Pyridoxal phosphate.jpg]] © Wikipedia</center> <br/> | ||
| Line 74: | Line 75: | ||
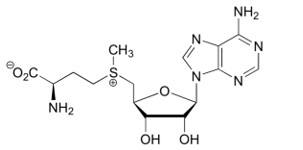
*Binding of SAM to the Bateman module destabilizes the interactions which sustain the tetramer structure and thus triggers the '''dissociation of the tetrameric structure''' into two dimers. | *Binding of SAM to the Bateman module destabilizes the interactions which sustain the tetramer structure and thus triggers the '''dissociation of the tetrameric structure''' into two dimers. | ||
| - | *SAM fixation on the C-terminal regulatory domain triggers the small rotation (or at least displacement) of the CBS1 and CBS2 of the Bateman module, thus distabilizing its interactions (hydrophobic interactions and hydrogen bounds) with the catalytic core of the other monomer. As a result, '''the Bateman module moves away from the catalytic core''' (this movement is facilitated | + | *SAM fixation on the C-terminal regulatory domain triggers the small rotation (or at least displacement) of the CBS1 and CBS2 of the Bateman module, thus distabilizing its interactions (hydrophobic interactions and hydrogen bounds) with the catalytic core of the other monomer. As a result, '''the Bateman module moves away from the catalytic core''' (this movement is facilitated since the linker region is long enough and made of flexible residues). <scene name='71/719867/Scene_5/1'>Loops 145-148, 171-174 and 191-202</scene>, previously involved in maintaining the close conformation through their interaction with the Bateman module, then relax and therefore '''allow accessibility to the catalytic site''' (open conformation). |
*SAM release leads to the return to the native inactive close conformation. | *SAM release leads to the return to the native inactive close conformation. | ||
| Line 82: | Line 83: | ||
A tight control of cystathionine β-synthase level and activity is crucial for optimal cognitive function. | A tight control of cystathionine β-synthase level and activity is crucial for optimal cognitive function. | ||
| - | Due to the fact that the CBS gene is located on chromosome 21 (21q22.3), an '''overexpression of CBS''' is observed in patients with '''Down Syndrome''', or trisomy 21. <br/> Hence, Down Syndrome is characterized by high plasma levels of cystathionine and cysteine and this disorder is typically associated with mental retardation. | + | Due to the fact that the CBS gene is located on chromosome 21 (21q22.3), an '''overexpression of CBS''' is observed in patients with '''Down Syndrome''', or trisomy 21. <br/> Hence, Down Syndrome is characterized by high plasma levels of cystathionine and cysteine and this disorder is typically associated with mental retardation. <ref>PMID: 23422941</ref> |
| - | On the contrary, a '''reduced activity of CBS''' leads to '''homocystinuria'''. This disorder is inherited in an autosomal recessive pattern and is caused by loss-of-function mutations in the hCBS gene. Those mutations interfere with the activation of CBS. <br/> Thus, Homocystinuria is characterized by high plasma levels of the toxic amino acid homocysteine and infants who develop this disease may have difficulties to grow and gain weight accompanied by mental retardation. | + | On the contrary, a '''reduced activity of CBS''' leads to '''homocystinuria'''. This disorder is inherited in an autosomal recessive pattern and is caused by loss-of-function mutations in the hCBS gene. Those mutations interfere with the activation of CBS. <br/> Thus, Homocystinuria is characterized by high plasma levels of the toxic amino acid homocysteine and infants who develop this disease may have difficulties to grow and gain weight accompanied by mental retardation. <ref>PMID: 20301697</ref> |
| Line 90: | Line 91: | ||
== References == | == References == | ||
<references/> | <references/> | ||
| + | |||
| + | <br/> | ||
| + | |||
| + | [[User:Alice Blondel.|Alice Blondel.]] 23:54, 30 January 2016 (IST) [[User:Mathilde Errard.|Mathilde Errard.]] 23:48, 30 January 2016 (IST) [[User:Antonin Tidu|Antonin Tidu]] 23:51, 30 January 2016 (IST) | ||
Current revision
| This Sandbox is Reserved from 15/12/2015, through 15/06/2016 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1120 through Sandbox Reserved 1159. |
To get started:
More help: Help:Editing |
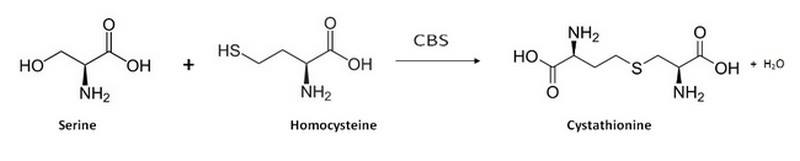
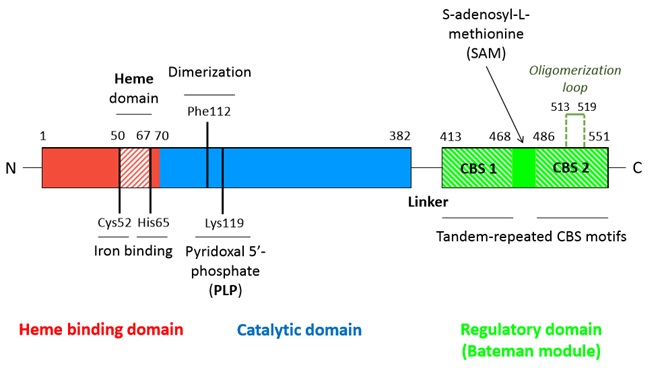
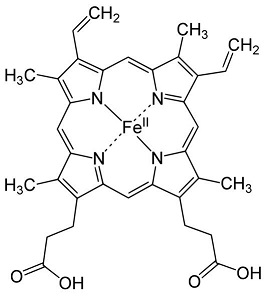
Human cystathionine β-synthase (hCBS)
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Meier M, Janosik M, Kery V, Kraus JP, Burkhard P. Structure of human cystathionine beta-synthase: a unique pyridoxal 5'-phosphate-dependent heme protein. EMBO J. 2001 Aug 1;20(15):3910-6. PMID:11483494 doi:http://dx.doi.org/10.1093/emboj/20.15.3910
- ↑ Regnier V, Billard JM, Gupta S, Potier B, Woerner S, Paly E, Ledru A, David S, Luilier S, Bizot JC, Vacano G, Kraus JP, Patterson D, Kruger WD, Delabar JM, London J. Brain phenotype of transgenic mice overexpressing cystathionine beta-synthase. PLoS One. 2012;7(1):e29056. doi: 10.1371/journal.pone.0029056. Epub 2012 Jan 12. PMID:22253703 doi:http://dx.doi.org/10.1371/journal.pone.0029056
- ↑ McCorvie TJ, Kopec J, Hyung SJ, Fitzpatrick F, Feng X, Termine D, Strain-Damerell C, Vollmar M, Fleming J, Janz JM, Bulawa C, Yue WW. Inter-Domain Communication Of Human Cystathionine Beta Synthase: Structural Basis Of S-Adenosyl-L-Methionine Activation. J Biol Chem. 2014 Oct 21. pii: jbc.M114.610782. PMID:25336647 doi:http://dx.doi.org/10.1074/jbc.M114.610782
- ↑ 4.0 4.1 4.2 Ereno-Orbea J, Majtan T, Oyenarte I, Kraus JP, Martinez-Cruz LA. Structural basis of regulation and oligomerization of human cystathionine beta-synthase, the central enzyme of transsulfuration. Proc Natl Acad Sci U S A. 2013 Sep 16. PMID:24043838 doi:10.1073/pnas.1313683110
- ↑ Miles EW, Kraus JP. Cystathionine beta-synthase: structure, function, regulation, and location of homocystinuria-causing mutations. J Biol Chem. 2004 Jul 16;279(29):29871-4. Epub 2004 Apr 15. PMID:15087459 doi:http://dx.doi.org/10.1074/jbc.R400005200
- ↑ http://www.ebi.ac.uk/interpro/entry/IPR004625
- ↑ https://pubchem.ncbi.nlm.nih.gov/compound/pyridoxal_phosphate#section=Top
- ↑ 8.0 8.1 Ereno-Orbea J, Majtan T, Oyenarte I, Kraus JP, Martinez-Cruz LA. Structural insight into the molecular mechanism of allosteric activation of human cystathionine beta-synthase by S-adenosylmethionine. Proc Natl Acad Sci U S A. 2014 Sep 16;111(37):E3845-52. doi:, 10.1073/pnas.1414545111. Epub 2014 Sep 2. PMID:25197074 doi:http://dx.doi.org/10.1073/pnas.1414545111
- ↑ Megarbane A, Noguier F, Stora S, Manchon L, Mircher C, Bruno R, Dorison N, Pierrat F, Rethore MO, Trentin B, Ravel A, Morent M, Lefranc G, Piquemal D. The intellectual disability of trisomy 21: differences in gene expression in a case series of patients with lower and higher IQ. Eur J Hum Genet. 2013 Nov;21(11):1253-9. doi: 10.1038/ejhg.2013.24. Epub 2013 Feb, 20. PMID:23422941 doi:http://dx.doi.org/10.1038/ejhg.2013.24
- ↑ Picker JD, Levy HL. Homocystinuria Caused by Cystathionine Beta-Synthase Deficiency PMID:20301697
Alice Blondel. 23:54, 30 January 2016 (IST) Mathilde Errard. 23:48, 30 January 2016 (IST) Antonin Tidu 23:51, 30 January 2016 (IST)