We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
MDM2
From Proteopedia
(Difference between revisions)
| (7 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
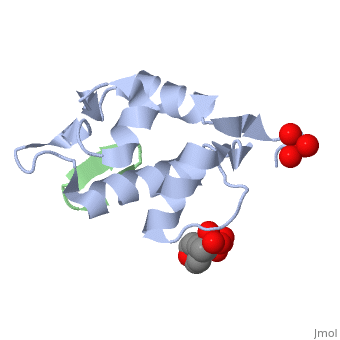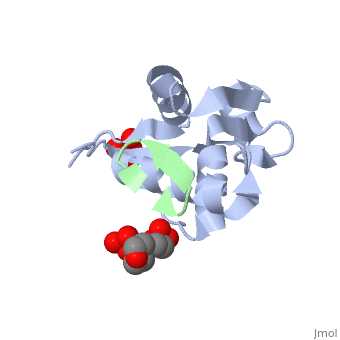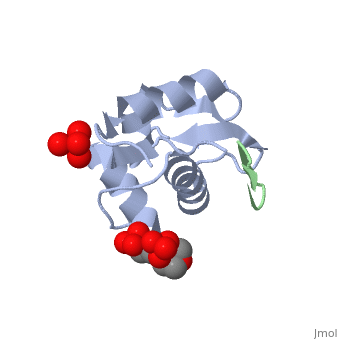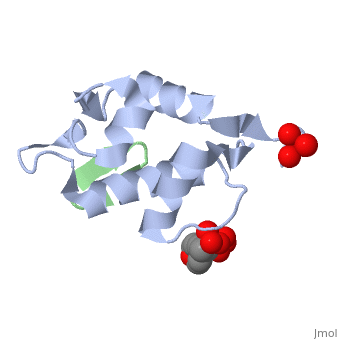
| - | + | <StructureSection load='2axi' size='350' side='right' scene='' caption='Human MDM2 (grey) complex with β-hairpin peptide (green), sulfate and morpholino-propane sulfonic acid [[1ycq]]'> | |
| - | + | __TOC__ | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | ==Function== | ||
The '''MDM2''' or '''E3 ubiquitin-protein ligase MDM2''' oncoprotein is a cellular inhibitor of the [[P53|p53 tumor suppressor]] in that it can bind the transactivation domain of p53 and downregulate its ability to activate transcription. In certain [[Cancer|cancers]], MDM2 amplification is a common event and contributes to the inactivation of p53. The crystal structure of the 109-residue amino-terminal domain of MDM2 bound to 109-residue transactivation domain peptide of p53 revealed that MDM2 has a <scene name='Matt_Routh_sandbox/Hydrophobic_cavity/1'>deep hydrophobic cleft</scene> on which the p53 peptide binds as an amphipathic alpha helix. The interface relies on the steric complementarity between the MDM2 cleft and the hydrophobic face of the p53 alpha helix and, in particular, on a triad of p53 amino acids-Phe19, Trp23, and Leu26-which insert <scene name='Matt_Routh_sandbox/Connection_to_p53/5'>deep inside</scene> the MDM2 cleft. These same p53 residues are also involved in transactivation, supporting the hypothesis that MDM2 inactivates p53 by concealing its transactivation domain. The structure also suggests that the amphipathic alpha helix may be a common structural motif in the binding of a diverse family of transactivation factors to the TATA-binding protein-associated factors.<ref name="Kussie">PMID:8875929</ref> | The '''MDM2''' or '''E3 ubiquitin-protein ligase MDM2''' oncoprotein is a cellular inhibitor of the [[P53|p53 tumor suppressor]] in that it can bind the transactivation domain of p53 and downregulate its ability to activate transcription. In certain [[Cancer|cancers]], MDM2 amplification is a common event and contributes to the inactivation of p53. The crystal structure of the 109-residue amino-terminal domain of MDM2 bound to 109-residue transactivation domain peptide of p53 revealed that MDM2 has a <scene name='Matt_Routh_sandbox/Hydrophobic_cavity/1'>deep hydrophobic cleft</scene> on which the p53 peptide binds as an amphipathic alpha helix. The interface relies on the steric complementarity between the MDM2 cleft and the hydrophobic face of the p53 alpha helix and, in particular, on a triad of p53 amino acids-Phe19, Trp23, and Leu26-which insert <scene name='Matt_Routh_sandbox/Connection_to_p53/5'>deep inside</scene> the MDM2 cleft. These same p53 residues are also involved in transactivation, supporting the hypothesis that MDM2 inactivates p53 by concealing its transactivation domain. The structure also suggests that the amphipathic alpha helix may be a common structural motif in the binding of a diverse family of transactivation factors to the TATA-binding protein-associated factors.<ref name="Kussie">PMID:8875929</ref> | ||
| Line 41: | Line 18: | ||
==3D structures of MDM2== | ==3D structures of MDM2== | ||
| + | [[MDM2 3D structures]] | ||
| - | + | </StructureSection> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==Additional Resources== | ==Additional Resources== | ||
Current revision
| |||||||||||
Additional Resources
For additional information, see: Cancer
Reference
- ↑ Kussie PH, Gorina S, Marechal V, Elenbaas B, Moreau J, Levine AJ, Pavletich NP. Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain. Science. 1996 Nov 8;274(5289):948-53. PMID:8875929
- ↑ 2.0 2.1 2.2 Pollock RE, Lang A, El-Naggar AK, Radinsky R, Hung MC. Enhanced MDM2 Oncoprotein Expression in Soft Tissue Sarcoma: Several Possible Regulatory Mechanisms. Sarcoma. 1997;1(1):23-9. PMID:18521197 doi:10.1080/13577149778443
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Matt Routh, David Canner, Joel L. Sussman, Yousuf Bahrami, Alexander Berchansky