This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Titin
From Proteopedia
(Difference between revisions)
| (8 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
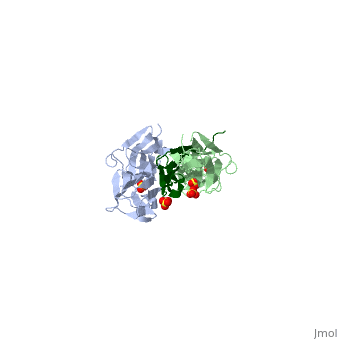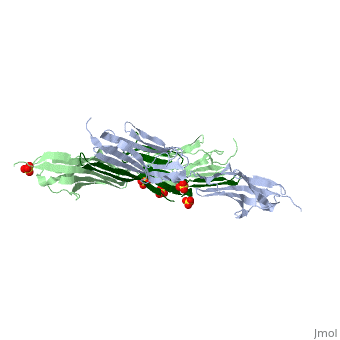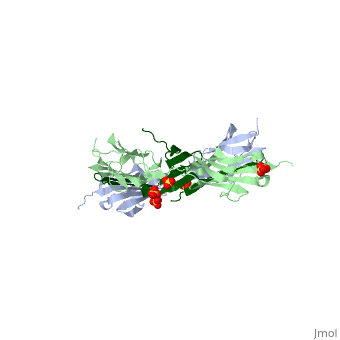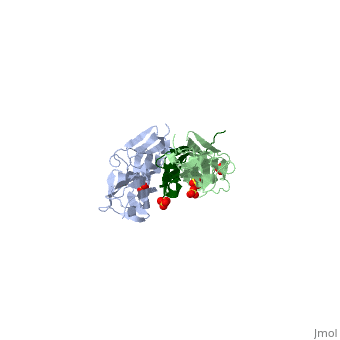
| - | <StructureSection load="1ya5" size=" | + | <StructureSection load="1ya5" size="350" caption="Human titin dimer residues 1-196 (grey and pale green) complex with telethonin (dark green) and sulfate, [[1ya5]]"> |
| - | [[Titin]] (TTN) is the largest known protein. The human TTN contains 34,350 residues. It is responsible for the passive elasticity of muscle. It has 244 domains connected by unstructured regions. The domains unfold when TTN is stretched. Additional details in<br /> | + | [[Titin]] (TTN) is the largest known protein. The human TTN contains 34,350 residues. It is responsible for the passive elasticity of muscle. It has 244 domains connected by unstructured regions. The domains unfold when TTN is stretched. <scene name='41/417484/Cv/1'>Human titin dimer residues 1-196 complex with telethonin</scene> ([[1ya5]]). Additional details in<br /> |
*[[Titin Structure & Function]]<br /> | *[[Titin Structure & Function]]<br /> | ||
*[[Group:MUZIC:Titin]]. | *[[Group:MUZIC:Titin]]. | ||
| Line 12: | Line 12: | ||
==Introduction== | ==Introduction== | ||
| - | Titin, also known as connectin, is an elastic and approximately 3,6 MDalton large protein which assembles itself to protein filaments. It is made of more than 30000 amino acids and includes 320 protein domains and therefore is known as the largest human protein. | + | '''Titin''', also known as '''connectin''', is an elastic and approximately 3,6 MDalton large protein which assembles itself to protein filaments. It is made of more than 30000 amino acids and includes 320 protein domains and therefore is known as the largest human protein. |
It is a part of the [http://en.wikipedia.org/wiki/Sarcomere sarcomere], the smallest functional unit in the striated muscles. Tintins task in the sarcomere is to center the [http://en.wikipedia.org/wiki/Myosin myosinheads] between the [http://en.wikipedia.org/wiki/Actin actin] filaments and to re-establish the unstreched mode. | It is a part of the [http://en.wikipedia.org/wiki/Sarcomere sarcomere], the smallest functional unit in the striated muscles. Tintins task in the sarcomere is to center the [http://en.wikipedia.org/wiki/Myosin myosinheads] between the [http://en.wikipedia.org/wiki/Actin actin] filaments and to re-establish the unstreched mode. | ||
| Line 40: | Line 40: | ||
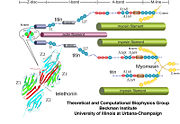
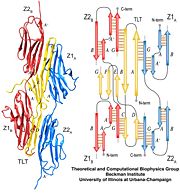
As seen in the image (Z1Z2 / Telethonin complex), the major force enduring component of this complex is an elaborate intermolecular hydrogen bonding network formed across <scene name='2a38/Test/2'>β-strand</scene> among telethonin and Z1Z2 domains, and not intramolecularly among termini β-strands of individual Z1 or Z2 domains. This shift to a stronger force enduring interface reduces the possibility of unraveling the individual Ig-domains, thus stabilizing the complex. This demonstrates how <scene name='2a38/Test/2'>β-strand</scene> cross-linking via [http://en.wikipedia.org/wiki/Hydrogen_bonds hydrogen bonds] serves as an important mechanism. It functions as a molecular adhesive, increasing the ability of protein complexes to resist against mechanical stress. | As seen in the image (Z1Z2 / Telethonin complex), the major force enduring component of this complex is an elaborate intermolecular hydrogen bonding network formed across <scene name='2a38/Test/2'>β-strand</scene> among telethonin and Z1Z2 domains, and not intramolecularly among termini β-strands of individual Z1 or Z2 domains. This shift to a stronger force enduring interface reduces the possibility of unraveling the individual Ig-domains, thus stabilizing the complex. This demonstrates how <scene name='2a38/Test/2'>β-strand</scene> cross-linking via [http://en.wikipedia.org/wiki/Hydrogen_bonds hydrogen bonds] serves as an important mechanism. It functions as a molecular adhesive, increasing the ability of protein complexes to resist against mechanical stress. | ||
| - | == | + | ==Disease== |
| - | + | See [[Titin related diseases]]. | |
| + | |||
| + | == 3D Structures of Titin == | ||
| - | [[ | + | [[Titin 3D structures]] |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==Additional Resources== | ==Additional Resources== | ||
See: [[Titin Structure & Function]] for additional information <br /> | See: [[Titin Structure & Function]] for additional information <br /> | ||
Current revision
| |||||||||||
References
- http://www.ncbi.nlm.nih.gov:80/pmc/articles/PMC1948054/?tool=pmcentrez
- http://www.ks.uiuc.edu/Research/z1z2/
- http://www.ks.uiuc.edu/Research/telethonin/
- http://de.wikipedia.org/wiki/Titin
Created with the participation of Anton Schmidt, Wolfgang Hermann.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, David Canner, Jaime Prilusky
Categories: Topic Page | Homo sapiens | Demirel, M. | Marino, M. | Mayans, O. | Muhle-Goll, C. | Svergun, D. | Titin | Z1z2