This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 1167
From Proteopedia
(Difference between revisions)
| (52 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
{{Sandbox_Reserved_CH462_Central_Metabolism}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | {{Sandbox_Reserved_CH462_Central_Metabolism}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
==Class B Human Glucagon G-Protein Coupled Receptor== | ==Class B Human Glucagon G-Protein Coupled Receptor== | ||
| - | <StructureSection load='4l6r' size='340' side='right' caption='Human Glucagon Class B GPCR ( 7tm PDB: [[4l6r]], ECD PDB: [[4ers]])' scene='72/721538/Glucagon_receptor/ | + | <StructureSection load='4l6r' size='340' side='right' caption='Human Glucagon Class B GPCR ( 7tm PDB: [[4l6r]], ECD PDB: [[4ers]])' scene='72/721538/Glucagon_receptor/2'> |
| - | == | + | == Introduction == |
| - | + | The '''human glucagon receptor''' ('''GCGR''') is one of 15 secretin-like, or Class B, [https://en.wikipedia.org/wiki/G_protein%E2%80%93coupled_receptor G-protein coupled receptors] (GPCRs). Like other GPCRs, it has a <scene name='72/721538/7tm_labeled_helicies/3'>7 trans-membrane </scene> helical domain and a globular N-terminus <scene name='72/721538/Ecd/2'>extracellular domain</scene> (ECD). | |
== Function == | == Function == | ||
| - | The | + | The glucagon receptor plays an important role in glucose homeostasis. During times of fasting (or low blood sugar) the pancreas produces glucagon to activate the GCGR in the liver. The [http://www.nature.com/nature/journal/v499/n7459/fig_tab/nature12393_F5.html binding of glucagon] to the extracellular side of GCGR leads to the activation of the receptor. On the intracellular side, upon this activation, a guanine diphosphate (GDP) is exchanged for a guanine triphosphate (GTP) - which, in turn, activates adenylate cyclase. Converting adenosine triphosphate (ATP) into cyclic adenosine monophosphate (cAMP), adenylate cyclase initiates protein kinase A (PKA) activity, which releases glucose into the blood stream. Overall, activation of the GCGR elevates blood sugar levels. |
| Line 16: | Line 16: | ||
=== Class B vs. Class A === | === Class B vs. Class A === | ||
| - | + | Like all classes of glucagon receptors, which include class A ([https://en.wikipedia.org/wiki/Rhodopsin-like_receptors rhodopsin-like]), B (secretin-like), and [https://en.wikipedia.org/wiki/Class_C_GPCR C] ([https://en.wikipedia.org/wiki/Metabotropic_glutamate_receptor metabotropic glutamate]), GCGR has a 7tm domain. While class B receptors do share characteristics with class C receptors, they are more similar to class A receptors. Class B receptors and class A receptors share less than 15% sequence [https://en.wikipedia.org/wiki/Homology_(biology) homology]; however, they do share similar [https://en.wikipedia.org/wiki/Signal_transduction signal transduction] mechanisms as well as the 7tm domain. The orientations and positions of the 7tm helices are also conserved between both classes of glucagon receptors<ref name ='structure_article'>PMID:23863937</ref>. | |
| - | + | However, one particular difference between class A receptors and class B receptors is an inward shift of the intracellular component of Helix VII. In class A receptors this inward shift is instrumental in receptor activation, yet in class B receptors it remains unclear what role this shift plays<ref name ='structure_article'>PMID:23863937</ref>. | |
| + | |||
| + | In contrast to class A glucagon receptors which have a [https://en.wikipedia.org/wiki/Proline proline] kink, in all [https://en.wikipedia.org/wiki/Secretin_receptor_family secretin-like class B glucagon receptors] a [https://en.wikipedia.org/wiki/Glycine Glycine] at position 393 in Helix VII allows for a <scene name='72/721537/Gly_393_helical_bend/3'>helical bend</scene>. This glycine helical bend is fully [https://en.wikipedia.org/wiki/Conserved_sequence conserved] in all secretin-like class B receptors. | ||
| - | + | Another important structural component found in all secretin-like class B receptors are the two conserved [https://en.wikipedia.org/wiki/Salt_bridge_%28protein_and_supramolecular%29 salt bridges] found between [https://en.wikipedia.org/wiki/Arginine Arg] 346 and [https://en.wikipedia.org/wiki/Glutamic_acid Glu] 406 and Arg 173 and Glu 406. These <scene name='72/721537/Salt_bridges/2'>salt bridges</scene> are a distinct feature of class B receptors because their interaction results in the distinct stalk found only in class B receptors<ref name ='structure_article'>PMID:23863937</ref>. | |
| - | + | While interface interactions between helices VI, V, and III, are not unique to class B receptors because certain homologous and even conserved residues exist in class A receptors (like [https://en.wikipedia.org/wiki/Tyrosine Tyr] 239 and Leu 358), as a part of the interface <scene name='72/721537/Helical_interactions_vi-v-iii/2'>stabilization</scene> between helices VI, V, and III, a Class B-specific [https://en.wikipedia.org/wiki/Hydrogen_bond hydrogen bond] occurs between [https://en.wikipedia.org/wiki/Asparagine Asn] 318 of Helix V and [https://en.wikipedia.org/wiki/Leucine Leu] 242 of Helix III <ref name ='structure_article'>PMID:23863937</ref>. | |
=== GCGR-Specific Traits === | === GCGR-Specific Traits === | ||
==== Helix I Stalk Region ==== | ==== Helix I Stalk Region ==== | ||
| - | The tip of Helix I extends above the cell membrane into the extracellular space creating a <scene name='72/721538/Helix_i/14'> stalk region</scene>. This region is longer than any other class of GPCR and extends | + | The tip of Helix I extends above the cell membrane into the extracellular space creating a <scene name='72/721538/Helix_i/14'> stalk region</scene>. This region is longer than any other class of GPCR and extends three α-helical turns above the plane of the membrane. It is proposed that the stalk helps to capture the glucagon peptide and facilitates it's insertion into the 7tm<ref name ='structure_article'>PMID:23863937</ref>. |
==== Intracellular Helix VIII ==== | ==== Intracellular Helix VIII ==== | ||
| - | The GCGR also contains an intracellular Helix VIII that is comprised of roughly 20 amino acids at the C-terminal end. This helix tilts approximately 25 degrees away from the membrane - the corresponding position in | + | The GCGR also contains an intracellular Helix VIII that is comprised of roughly 20 amino acids at the C-terminal end. This helix tilts approximately 25 degrees away from the membrane - the corresponding position in class A receptors are turned toward the membrane<ref name="structure_article" />. Although researchers are not entirely sure of its function, this helix is completely conserved in class B structures. |
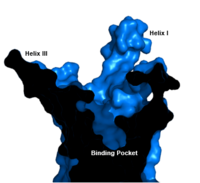
==== Binding Pocket ==== | ==== Binding Pocket ==== | ||
| - | The | + | [[Image:Labeled_Binding_Pocket.png|200 px|left|thumb|'''Figure 1. GCGR Binding Pocket.''' A cross-section of the GCGR binding pocket shows its width and depth]]The class B GPCR has the widest and longest <scene name='72/721538/Binding_pocket/1'>binding pocket</scene> of all other classes of GPCRs. The distance between the EC tips of Helicies II and VI as well as between the tips of Helicies III and VII are some of the largest among the GPCRs<ref name="structure_article" />. As a result, the [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3820480/bin/nihms495648f2.jpg binding cavity] of GCGR is located deeper inside the receptor, meaning glucagon binds much closer to the cell membrane. |
====Other Unique Structural Features ==== | ====Other Unique Structural Features ==== | ||
| - | An important interface stabilization interaction between | + | An important interface stabilization interaction between Helices I and VII occurs between [https://en.wikipedia.org/wiki/Serine Ser] 152 of Helix I and Ser 390 of Helix VII. Due to their close proximity to one another, they form an important <scene name='72/721537/Ser-ser_hydrogen_bond/3'>hydrogen bond</scene> which stabilizes the structure of GCGR. Mutations to the homologous residues Ser 135 and Ser 392 have been shown to alter receptor signaling in [https://en.wikipedia.org/wiki/Glucagon-like_peptide_1_receptor glucagon-like peptide-1 receptor] (GLP1R). |
== Glucagon Binding == | == Glucagon Binding == | ||
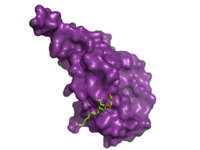
| - | Research has shown that | + | [[Image:ECD_bound_to_glucagon.png|200 px|left|thumb|'''Figure 2. Bound Molecule of Glucagon.''' A molecule of glucagon is shown bound to the GCGR's ECD (shown in magenta)]]Research has shown that class B GCPRs exist in either an [http://www.nature.com/ncomms/2015/150731/ncomms8859/fig_tab/ncomms8859_F3.html open or closed conformation] differentiating between the receptor's active and inactive states. The active, or open conformation, is characterized by an intracellular outward movement of <scene name='72/721538/Helix_v_and_vi/1'>helicies V and VI</scene> (breaking hydrogen bonds between <scene name='72/721538/Arg173-ser350_h_bond/1'>Arg173-Ser350</scene> and <scene name='72/721538/Arg173-ser350_h_bond/2'>Glu245-Thr351</scene>)<ref name='therapeutic_article'>DOI 10.1016/j.tips.2013.11.001</ref> and an extracellular rotation of the ECD until it is almost perpendicular to the membrane surface <ref name ='conformation_article'>PMID:26227798 </ref>. While the stalk region of Helix I helps to facilitate the motion of the ECD, intracellular G-protein coupling and extracellular glucagon binding stabilized this active state. In the abscence of glucagon, however, the GCGR adopts a closed conformation in which all three of the extracellular loops of the 7tm (<scene name='72/721538/Ecls/1'>ECL1, ECL2, and ECL3</scene>) can interact with the ECD <ref name ='conformation_article'>PMID:26227798 </ref>. In this closed state, the ECD covers the extracellular surface of the 7tm. To transition between states, the ECD rotates and moves down towards the 7tm domain. This transition mechanism is consistent with the "two-domain" binding mechanism of class B GCPRs in which (1) the C-terminus of the ligand first binds to the ECD allowing (2) the N-terminus of the ligand to interact with the 7tm and activate the protein <ref name='therapeutic_article'>DOI 10.1016/j.tips.2013.11.001</ref>. |
| - | + | ||
| - | + | ||
| - | + | ||
| - | This transition mechanism is consistent with the "two-domain" binding mechanism of | + | |
== Clinical Relevance == | == Clinical Relevance == | ||
| - | Because of GCGR's role in glucose homeostasis, | + | Because of GCGR's role in glucose homeostasis, GCGRis a potential drug target for [https://en.wikipedia.org/wiki/Diabetes_mellitus_type_2 Type 2 diabetes]. Specifically, molecules that antagonize the glucagon receptor may be able to lower blood sugar levels. Among experimental treatments, two antibodies, mAb1 and mAb23, target the ECD domain of the GCGR interrupting glucagon binding<ref name='other_article'>PMID:22908259</ref>. While the entire cleft of the ECD is blocked by mAb1, mAb3 blocks glucagon binding by stabilizing a conformation of the ECD that promotes receptor inactivation <ref name='other_article'>PMID:22908259</ref>. Another antibody, mAb7, inhibits GCGR allosterically<ref>PMID:24189067</ref>. Through binding to a site outside of the binding pocket, mAb7 inhibits the receptor without interacting with essential glucagon binding residues. Disrupting the normal interactions between the ECD and the 7tm domains, these antibodies inhibit the receptor's function and help to lower blood glucose level. |
| + | As GCGR holds great promise as a therapeutic target, there are currently three drugs under development that are designed to treat Type 2 Diabetes by targeting the human glucagon receptor<ref name='therapeutic_article'>DOI 10.1016/j.tips.2013.11.001</ref>. Although they are still in phase I of their clinical trials, preliminary research has shown promising results<ref>DOI 10.2174/1573399810666141224121927</ref>. | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
| This Sandbox is Reserved from Jan 11 through August 12, 2016 for use in the course CH462 Central Metabolism taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1160 through Sandbox Reserved 1184. |
To get started:
More help: Help:Editing |
Class B Human Glucagon G-Protein Coupled Receptor
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Siu FY, He M, de Graaf C, Han GW, Yang D, Zhang Z, Zhou C, Xu Q, Wacker D, Joseph JS, Liu W, Lau J, Cherezov V, Katritch V, Wang MW, Stevens RC. Structure of the human glucagon class B G-protein-coupled receptor. Nature. 2013 Jul 25;499(7459):444-9. doi: 10.1038/nature12393. Epub 2013 Jul 17. PMID:23863937 doi:10.1038/nature12393
- ↑ 2.0 2.1 2.2 Hollenstein K, de Graaf C, Bortolato A, Wang MW, Marshall FH, Stevens RC. Insights into the structure of class B GPCRs. Trends Pharmacol Sci. 2014 Jan;35(1):12-22. doi: 10.1016/j.tips.2013.11.001. Epub, 2013 Dec 18. PMID:24359917 doi:http://dx.doi.org/10.1016/j.tips.2013.11.001
- ↑ 3.0 3.1 Yang L, Yang D, de Graaf C, Moeller A, West GM, Dharmarajan V, Wang C, Siu FY, Song G, Reedtz-Runge S, Pascal BD, Wu B, Potter CS, Zhou H, Griffin PR, Carragher B, Yang H, Wang MW, Stevens RC, Jiang H. Conformational states of the full-length glucagon receptor. Nat Commun. 2015 Jul 31;6:7859. doi: 10.1038/ncomms8859. PMID:26227798 doi:http://dx.doi.org/10.1038/ncomms8859
- ↑ 4.0 4.1 Koth CM, Murray JM, Mukund S, Madjidi A, Minn A, Clarke HJ, Wong T, Chiang V, Luis E, Estevez A, Rondon J, Zhang Y, Hotzel I, Allan BB. Molecular basis for negative regulation of the glucagon receptor. Proc Natl Acad Sci U S A. 2012 Sep 4;109(36):14393-8. Epub 2012 Aug 20. PMID:22908259 doi:http://dx.doi.org/10.1073/pnas.1206734109
- ↑ Mukund S, Shang Y, Clarke HJ, Madjidi A, Corn JE, Kates L, Kolumam G, Chiang V, Luis E, Murray J, Zhang Y, Hotzel I, Koth CM, Allan BB. Inhibitory mechanism of an allosteric antibody targeting the glucagon receptor. J Biol Chem. 2013 Nov 4. PMID:24189067 doi:http://dx.doi.org/10.1074/jbc.M113.496984
- ↑ Mittermayer F, Caveney E, De Oliveira C, Gourgiotis L, Puri M, Tai LJ, Turner JR. Addressing unmet medical needs in type 2 diabetes: a narrative review of drugs under development. Curr Diabetes Rev. 2015;11(1):17-31. doi: 10.2174/1573399810666141224121927. PMID:25537454 doi:http://dx.doi.org/10.2174/1573399810666141224121927