We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 425
From Proteopedia
(Difference between revisions)
| (9 intermediate revisions not shown.) | |||
| Line 7: | Line 7: | ||
[[Student Projects for UMass Chemistry 423 Spring 2016]] | [[Student Projects for UMass Chemistry 423 Spring 2016]] | ||
| - | <StructureSection load='4uxq' size='350' side='right' caption='FGFR | + | <StructureSection load='4uxq' size='350' side='right' caption='The Ponatinib-FGFR complex is highly effective for treating CML ([[4uxq]])' scene=''> |
==Introduction== | ==Introduction== | ||
| Line 29: | Line 29: | ||
==Binding Interactions== | ==Binding Interactions== | ||
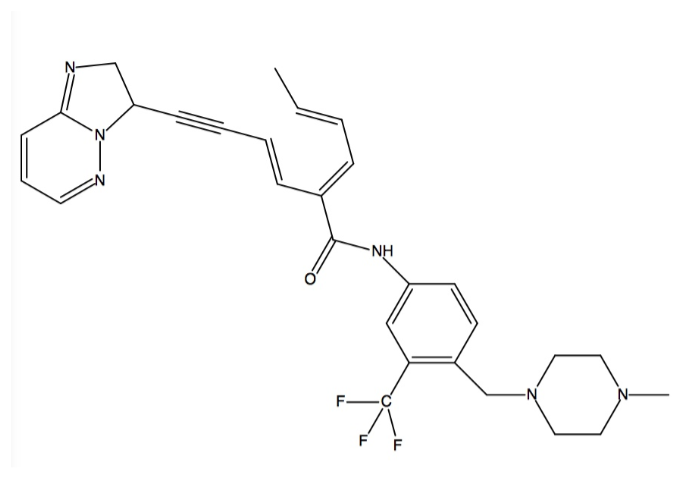
| - | Kinases are the largest drug targets | + | Kinases are the largest drug targets being tested. All kinases possess a biolobal fold that connects the N and C termini by a “hinge” that binds ATP. “Gatekeepers” are other residues that are in the hinge and alter binding capabilities. Mutations to gatekeepers are critical considerations in drug development because they can result in drug resistances<ref name="four">PMID: 25317566</ref>. Ponatinib can bind even in the presence of gatekeeper mutations, like T315I which accounts for 15-20% of all clinically observed mutations and is resistant to all previous generation drugs<ref name="five">PMID: 25219510</ref>. This class of inhibitors can bind deep within the hydrophobic and allosteric pocket that is only accessible in the <scene name='48/483882/Active_sitezoom/1'>DFG-out</scene> conformation (see color chart) which consists of Asp630, Phe631, and Glu632<ref name="four" />. |
<center><big>{{Template:ColorKey_N52C3Rainbow}}.</big></center> | <center><big>{{Template:ColorKey_N52C3Rainbow}}.</big></center> | ||
| - | + | Ponatinib binds to the ATP binding pocket between the N and C lobes to shift from the DFG-in to the DFG-out conformation. It spans from the <scene name='48/483882/Hinge/1'>hinge</scene> (<font color='cyan'><b>cyan</b></font>) to the front catalytic pocket. Three sites are engaged in the ATP binding cleft by ponatinib’s aromatic rings. First, imidazo[1,2b]pyridazine occupies the same space as the adenine ring of ATP and forms a <scene name='48/483882/Hingehbond/1'>hydrogen bond</scene> with the amide nitrogen atom of Ala553 (<font color='magenta'><b>magenta</b></font>) in the hinge<ref name="seven" />. Multiple triple bonds help the rest of ponatinib to move further in to the ATP binding pocket. Second, the methylphenyl group displaces the side chain of Lys503 and its aromatic ring binds to the hydrophobic pocket that is formed by Val550 (the <scene name='48/483882/Gatekeeper/1'>gatekeeper</scene> in <font color='yellow'><b>yellow</b></font>), and Met524. This allows Glu520 to hydrogen bond with the amide linkage between the aromatic rings in ponatinib. Third, Phe631 is replaced by ponatinib’s 3-trifluoromethylphenyl group. Asp630 becomes available for hydrogen bonding with the amide linkage between ponatinib’s aromatic rings and lets the piperazine ring hydrogen bond with the catalytic loop which forms the DFG-out conformation<ref name="seven" />. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==Additional Features== | ==Additional Features== | ||
| Line 45: | Line 41: | ||
The brand name for ponatinib is Iclusig. Iclusig received an accelerated approval grant through the Food and Drug Administration. It was mainly prescribed to patients suffering from Chronic Myeloid Leukemia or Acute Lymphoblastic Leukemia who did not make any progress with the first and second generation TKIs. However, the clinical trials data displayed a spike in adverse effects. These consequences include heart failure, stroke, coronary artery disease, loss of blood flow to body parts leading to amputation amongst other narrowing of blood vessels<ref>FDA Drug Safety Communication: FDA investigating leukemia drug Iclusig (ponatinib) after increased reports of serious blood clots in arteries and veins; Drug Safety and Availability; United States Food and Drug Administration (2013). Web. [http://www.fda.gov/Drugs/DrugSafety/ucm370945.htm]</ref>. | The brand name for ponatinib is Iclusig. Iclusig received an accelerated approval grant through the Food and Drug Administration. It was mainly prescribed to patients suffering from Chronic Myeloid Leukemia or Acute Lymphoblastic Leukemia who did not make any progress with the first and second generation TKIs. However, the clinical trials data displayed a spike in adverse effects. These consequences include heart failure, stroke, coronary artery disease, loss of blood flow to body parts leading to amputation amongst other narrowing of blood vessels<ref>FDA Drug Safety Communication: FDA investigating leukemia drug Iclusig (ponatinib) after increased reports of serious blood clots in arteries and veins; Drug Safety and Availability; United States Food and Drug Administration (2013). Web. [http://www.fda.gov/Drugs/DrugSafety/ucm370945.htm]</ref>. | ||
| - | FGFR-4 is abundantly present in human prostate cancer observed in vitro and in mouse model simulations<ref name="nine">PMID: 22573348</ref>. A <scene name='48/483882/Variant/ | + | FGFR-4 is abundantly present in human prostate cancer observed in vitro and in mouse model simulations<ref name="nine">PMID: 22573348</ref>. A <scene name='48/483882/Variant/9'>variant</scene> of FGFR-4 with Arg388 replacing Gly388 is implicated with increased human prostate cancer. This variation causes increased receptor stability and activation<ref name="ten">PMID:18670643</ref>. A study revealed that the <scene name='48/483882/Inhibition/1'>inhibition</scene> of FGFR-4 signaling completely curtailed prostate cancer cell lines that were responsible for tumor growth<ref name="nine">PMID: 22573348</ref>. Due to the significant results of diminished cell growth in treated tumors, targeting fibroblast growth factor signaling appears to provide a promising step towards combating aggressive prostate cancer. |
| Line 51: | Line 47: | ||
==Quiz Question 1== | ==Quiz Question 1== | ||
| - | Ponatinib is unique in it's ability to bind to the mutated BCR-ABL because of it's preference to shift to the DFG-out conformation. In theory, if a competitive inhibitor was created by nature to prevent Ponatinib from binding to BCR-ABL to further its drug resistance, what specific | + | Ponatinib is unique in it's ability to bind to the mutated BCR-ABL because of it's preference to shift to the DFG-out conformation. In theory, if a competitive inhibitor was created by nature to prevent Ponatinib from binding to BCR-ABL to further its drug resistance, what specific structural characteristics would the inhibitor need to possess? Consider the unique binding methods of Ponatinib and the <scene name='48/483882/Active_sitezoom/1'>DFG-out</scene> conformation. |
a. Small, fully conjugated aromatic system with no electronegative substituents, to prevent unwanted hydrogen bonding. | a. Small, fully conjugated aromatic system with no electronegative substituents, to prevent unwanted hydrogen bonding. | ||
Current revision
| This Sandbox is Reserved from January 19, 2016, through August 31, 2016 for use for Proteopedia Team Projects by the class Chemistry 423 Biochemistry for Chemists taught by Lynmarie K Thompson at University of Massachusetts Amherst, USA. This reservation includes Sandbox Reserved 425 through Sandbox Reserved 439. |
Fibroblast Growth Factor Receptor/Ponatinib (4uxq) [1]
by Julie Boshar, Emily Boyle, Nicole Kirby, Cory Thomas, Connor Walsh
Student Projects for UMass Chemistry 423 Spring 2016
| |||||||||||