Sandbox Reserved 434
From Proteopedia
(Difference between revisions)
| (3 intermediate revisions not shown.) | |||
| Line 28: | Line 28: | ||
==Additional Features== | ==Additional Features== | ||
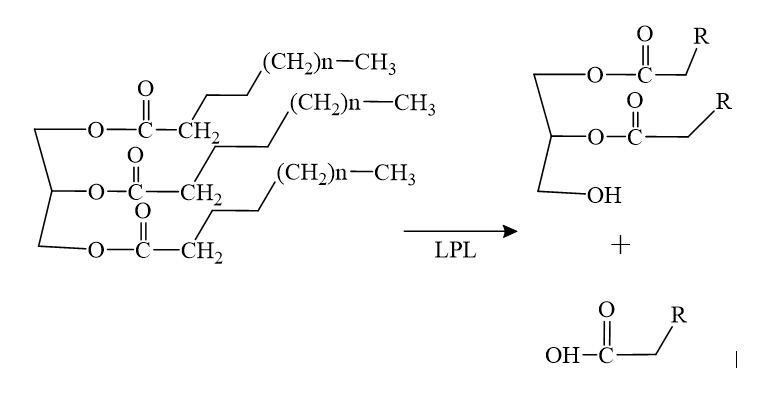
| - | The biological importance of pantetheinase is found in the products cysteamine and vitamin B5, formed from the hydrolysis reaction shown below. Cysteamine and vitamin B5 are key components in the synthesis of other necessary bio-molecules such as acetylcholine and coenzyme A. | + | The biological importance of pantetheinase is found in the products cysteamine and vitamin B5, formed from the hydrolysis reaction shown below. Cysteamine and vitamin B5 are key components in the synthesis of other necessary bio-molecules such as acetylcholine and coenzyme A. |
[[Image:rxn.png]] | [[Image:rxn.png]] | ||
===Inhibition=== | ===Inhibition=== | ||
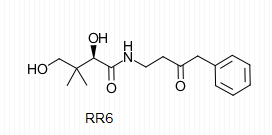
| - | The importance of pantetheinase stems from the vitality of the components of the reaction it catalyzes. It is for this reason that pantetheinase has been a promising point of research in the field of medicine. Pantetheine analogues known as pantothenamides have been shown to act as effective antibiotics that protect the body from bacterial intruders. The similarity of these analogues to pantetheine allows for the active sites on pantetheinase to catalyze their breakdown through hydrolysis. The administration of RR6 in the presence of pantetheinase and other antibiotic pantothenamides revealed that RR6 acts as a competitive inhibitor with great affinity for the active site Cys at <scene name='48/483891/Rr6/2'>residue 211</scene> on pantetheinase, thus preserving desired concentrations of the pantothenamides with antibiotic characteristics. | + | The importance of pantetheinase stems from the vitality of the components of the reaction it catalyzes. It is for this reason that pantetheinase has been a promising point of research in the field of medicine. Pantetheine analogues known as pantothenamides have been shown to act as effective antibiotics that protect the body from bacterial intruders. The similarity of these analogues to pantetheine allows for the active sites on pantetheinase to catalyze their breakdown through hydrolysis. The administration of RR6 in the presence of pantetheinase and other antibiotic pantothenamides revealed that RR6 acts as a competitive inhibitor with great affinity for the active site Cys at <scene name='48/483891/Rr6/2'>residue 211</scene> on pantetheinase, thus preserving desired concentrations of the pantothenamides with antibiotic characteristics. The RR6 inhibitor is shown below. |
[[Image:RR6.png]] | [[Image:RR6.png]] | ||
===Absence=== | ===Absence=== | ||
| - | Vitamin B5 is the recycled product of the hydrolysis of pantetheine and is a major substrate in the formation of coenzyme A (CoA). Without pantetheinase, the hydrolysis of | + | Vitamin B5 is the recycled product of the hydrolysis of pantetheine and is a major substrate in the formation of coenzyme A (CoA). Without pantetheinase, the hydrolysis of pantetheine would occur at a significantly slower rate and would therefore produce vitamin B5 and cysteamine at a slower rate. Lower concentrations of Vitamin B5 would result in inadequate prodution of CoA and therefore inadequate production of acetylcholine. Acetylcholine is a neurotransmitter that is needed for proper brain function which means an absence of pantetheinase could lead to neurological complications. |
| - | Cysteamine is important in the regulation of cystine levels in lysosomes. A lack of cysteamine due to the absence of pantetheinase would lead to a build up of lysosomal cystine, a disease known as cystinosis. | + | Cysteamine is important in the regulation of cystine levels in lysosomes. A lack of cysteamine due to the absence of pantetheinase would lead to a build up of lysosomal cystine, a disease known as cystinosis. |
==Quiz Question 1== | ==Quiz Question 1== | ||
| - | Pantetheinase exhibits an extremely similar sequence to the other proteins in the Vanin family. In addition, pantetheinase is also sequentially similar to this enzyme, which allows the body to utilize a certain vitamin: | + | Pantetheinase exhibits an extremely similar sequence to the other proteins in the Vanin family. In addition, <scene name='48/483891/Pantetheinase/1'>pantetheinase</scene> is also sequentially similar to this enzyme, which allows the body to utilize a certain vitamin: |
a. gastric lipase | a. gastric lipase | ||
| Line 52: | Line 52: | ||
d. pepsin | d. pepsin | ||
| + | |||
| + | |||
==See Also== | ==See Also== | ||
| Line 66: | Line 68: | ||
Drug Binding Site - Owen O'Connor | Drug Binding Site - Owen O'Connor | ||
| - | Additional Features - Nick | + | Additional Features - Nick Saint |
Quiz Question 1 - Tyler Russell | Quiz Question 1 - Tyler Russell | ||
==References== | ==References== | ||
| - | Yamada, Kazuhiro (2013). "Chapter 9. Cobalt: Its Role in Health and Disease". In Astrid Sigel, Helmut Sigel and Roland K. O. Sigel. Interrelations between Essential Metal Ions and Human Diseases. Metal Ions in Life Sciences 13. Springer. pp. 295–320.doi:10.1007/978-94-007-7500-8_9 | ||
<references/> | <references/> | ||
| + | [2] "Pantetheinase." VNN1. UniProt, n.d. Web. 10 Apr. 2016. | ||
| + | |||
| + | [3]Jansen, Patrick, and Joost Schalkwijk. "Chemical Biology Tools to Study Pantetheinases of the Vanin Family." Biochemical Society Transactions. Portland Press, n.d. Web. 10 Apr. 2016. | ||
| + | |||
| + | [4]"Cystinosis." Genetics Home Reference. U.S. National Library of Medicine, 8 Apr. 2016. Web. 10 Apr. 2016. | ||
Current revision
| This Sandbox is Reserved from January 19, 2016, through August 31, 2016 for use for Proteopedia Team Projects by the class Chemistry 423 Biochemistry for Chemists taught by Lynmarie K Thompson at University of Massachusetts Amherst, USA. This reservation includes Sandbox Reserved 425 through Sandbox Reserved 439. |
Pantetheinase (4CYG)[1]
by [Luke Schnitzler, Patrick Tonne, Owen O'Connor, Tyler Russell, Nicholas Sant]
Student Projects for UMass Chemistry 423 Spring 2016
| |||||||||||