Jumonji domain-containing protein
From Proteopedia
(Difference between revisions)
| (15 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
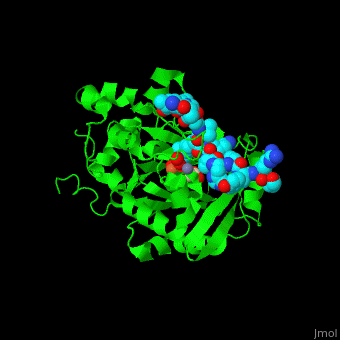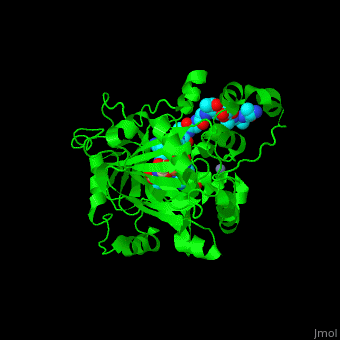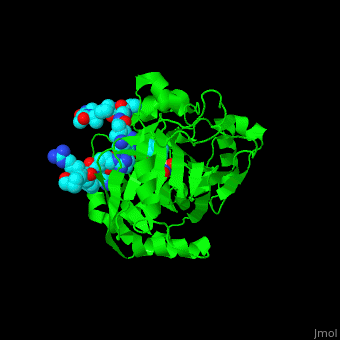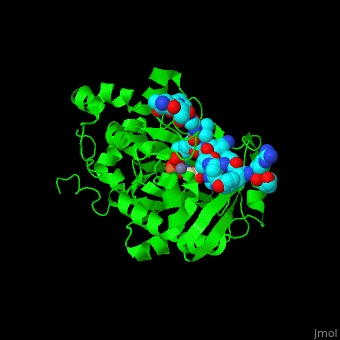
| - | <StructureSection load=' | + | <StructureSection load='' size='350' side='right' caption='Structure of human Jumonji domain-containing protein 2a catalytic domain complex with oxalylglycine, Fe+2 ion (orange), Zn+2 ion (grey) and histone H3 peptide (aqua) with trimethyllysine (magenta) (PDB code [[2p5b]]).' scene='59/596434/Cv/1'> |
| - | + | SEE ALSO [[Lysine-specific histone demethylase]] | |
== Function == | == Function == | ||
| - | *'''Jumonji domain-containing protein 2a''' (Jmjd2a) or '''KDM4A''' catalyzes the demethylation of trimethylated histone H3 at lysine residues 9 and 36 producing the dimethylated form. Jmjd2a domains contain: N-terminal, catalytic core (residues 1-350), 2 TUDOR domains which recognize dimethylated Arg (residues 895-1011) , 2 PHD-type zinc fingers and C-terminal domain. Jmjd2a is Fe+2 and α-ketoglutarate dependent. Methylation of histones in the chromatin is involved in regulation of gene activity, chromatin structure and epigenetic memory<ref>PMID:16677698</ref>.<br /> | + | *'''Jumonji domain-containing protein 2a''' (Jmjd2a) or '''KDM4A''' or '''Lysine-specific demethylase''' catalyzes the demethylation of trimethylated histone H3 at lysine residues 9 and 36 producing the dimethylated form. Jmjd2a domains contain: N-terminal, catalytic core (residues 1-350), 2 TUDOR domains which recognize dimethylated Arg (residues 895-1011) , 2 PHD-type zinc fingers and C-terminal domain. Jmjd2a is Fe+2 and α-ketoglutarate dependent. Methylation of histones in the chromatin is involved in regulation of gene activity, chromatin structure and epigenetic memory<ref>PMID:16677698</ref>.<br /> |
*'''Jumonji domain-containing protein 2b''' (Jmjd2b) or '''KDM4B''' and '''Jumonji domain-containing protein 2d''' (Jmjd2d) or '''KDM4D''' catalyze the demethylation of trimethylated histone H3 at lysine residues 9 producing the dimethylated form. <br /> | *'''Jumonji domain-containing protein 2b''' (Jmjd2b) or '''KDM4B''' and '''Jumonji domain-containing protein 2d''' (Jmjd2d) or '''KDM4D''' catalyze the demethylation of trimethylated histone H3 at lysine residues 9 producing the dimethylated form. <br /> | ||
*'''Jumonji domain-containing protein 3''' (Jmjd3) or '''KDM6B''' and '''Jumonji domain-containing protein 2d''' (Jmjd2d) or '''KDM4D''' catalyze the demethylation of trimethylated histone H3 at lysine residues 27 producing the dimethylated form<ref>PMID:22308433</ref>. <br /> | *'''Jumonji domain-containing protein 3''' (Jmjd3) or '''KDM6B''' and '''Jumonji domain-containing protein 2d''' (Jmjd2d) or '''KDM4D''' catalyze the demethylation of trimethylated histone H3 at lysine residues 27 producing the dimethylated form<ref>PMID:22308433</ref>. <br /> | ||
| Line 11: | Line 11: | ||
== Structural highlights == | == Structural highlights == | ||
| - | Jmjd2a uses <scene name='59/596434/Cv/ | + | Jmjd2a uses <scene name='59/596434/Cv/7'>9 residues for interaction with the methylated peptide</scene> and <scene name='59/596434/Cv/8'>4 residues are involved with binding to the peptide's trimethyllysine moiety</scene>. An <scene name='59/596434/Cv/9'>O2 molecules are recruited into the catalytic center</scene><ref>PMID:17567753</ref>. Water molecules shown as red spheres. |
| - | </ | + | |
| + | <scene name='59/596434/Cv/10'>Zn coordination site</scene>. | ||
==3D structures of jumonji domain-containing protein == | ==3D structures of jumonji domain-containing protein == | ||
| + | [[Jumonji domain-containing protein 3D structures]] | ||
| - | + | </StructureSection> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | }} | ||
== References == | == References == | ||
<references/> | <references/> | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
References
- ↑ Chen Z, Zang J, Whetstine J, Hong X, Davrazou F, Kutateladze TG, Simpson M, Mao Q, Pan CH, Dai S, Hagman J, Hansen K, Shi Y, Zhang G. Structural insights into histone demethylation by JMJD2 family members. Cell. 2006 May 19;125(4):691-702. Epub 2006 May 4. PMID:16677698 doi:http://dx.doi.org/10.1016/j.cell.2006.04.024
- ↑ Canovas S, Cibelli JB, Ross PJ. Jumonji domain-containing protein 3 regulates histone 3 lysine 27 methylation during bovine preimplantation development. Proc Natl Acad Sci U S A. 2012 Feb 14;109(7):2400-5. doi:, 10.1073/pnas.1119112109. Epub 2012 Jan 30. PMID:22308433 doi:http://dx.doi.org/10.1073/pnas.1119112109
- ↑ Lawrence P, Rai D, Conderino JS, Uddowla S, Rieder E. Role of Jumonji C-domain containing protein 6 (JMJD6) in infectivity of foot-and-mouth disease virus. Virology. 2016 Feb 18;492:38-52. doi: 10.1016/j.virol.2016.02.005. PMID:26896934 doi:http://dx.doi.org/10.1016/j.virol.2016.02.005
- ↑ Chen Z, Zang J, Kappler J, Hong X, Crawford F, Wang Q, Lan F, Jiang C, Whetstine J, Dai S, Hansen K, Shi Y, Zhang G. Structural basis of the recognition of a methylated histone tail by JMJD2A. Proc Natl Acad Sci U S A. 2007 Jun 26;104(26):10818-23. Epub 2007 Jun 13. PMID:17567753
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Joel L. Sussman, Jaime Prilusky