We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 430
From Proteopedia
(Difference between revisions)
| (7 intermediate revisions not shown.) | |||
| Line 17: | Line 17: | ||
As a member of a large class of G-protein-coupled receptors, P2Y12 is often an initial role player in signal transduction and cellular response due to external environmental factors. In the case of P2Y12, the receptor responds to ADP concentrations in the extracellular matrix and on a larger scale the blood stream. There is importance is understanding how P2Y12's structure receives ADP as an activator. In turn, knowledge of how P2Y12 is affected when properly inhibited can lead to improved drug design in terms of bioavailability, binding affinity, and effectiveness of inhibition. Ultimately, pharmaceuticals will be better able to prevent and treat cardiovascular diseases and medical conditions (thrombosis, hypercoagulable states) and more immediate dangers (stroke, embolism, and heart attacks). | As a member of a large class of G-protein-coupled receptors, P2Y12 is often an initial role player in signal transduction and cellular response due to external environmental factors. In the case of P2Y12, the receptor responds to ADP concentrations in the extracellular matrix and on a larger scale the blood stream. There is importance is understanding how P2Y12's structure receives ADP as an activator. In turn, knowledge of how P2Y12 is affected when properly inhibited can lead to improved drug design in terms of bioavailability, binding affinity, and effectiveness of inhibition. Ultimately, pharmaceuticals will be better able to prevent and treat cardiovascular diseases and medical conditions (thrombosis, hypercoagulable states) and more immediate dangers (stroke, embolism, and heart attacks). | ||
==Overall Structure== | ==Overall Structure== | ||
| - | The <scene name='48/483887/Secondary_structure/2'>secondary structure</scene> | + | P2Y12R is a 1 chain structure. The <scene name='48/483887/Secondary_structure/2'>secondary structure</scene> of P2Y12R consists of eight <font color='deeppink'>alpha helices</font>. Seven transmembrane alpha helices are tilted and in a bundle, while the carboxy-terminal helix VIII is parallel to the membrane bilayer. The <scene name='48/483887/Rainbow/3'>rainbow scene</scene> demonstrates how the chain goes from the <font color='blue'>N</font> to <font color='red'>C</font> termini with each helix being approximately one color each of the color scheme. |
| - | + | {{Template:ColorKey_N2CRainbow}} | |
| - | + | ||
| - | + | P2Y12R contains only one <scene name='48/483887/4ntj_disulfide_bond/1'>disulfide bond</scene> that connects the <font color='blue'>amino terminus</font> with <font color='darkorgange'>helix VII</font>. There are also two cholesterol molecules that are bound to two receptor molecules. As displayed in this <scene name='48/483887/Binding/2'>scene</scene>, one cholesterol molecule is bound to a receptor molecule between <font color='deepbluesky'>helix III</font> and <font color='lime'>helix V</font>. Another cholesterol molecule is bound to a receptor molecule shown <scene name='48/483887/Molecule/2'>here</scene> at the interface of <font color='blue'>helix I</font> and <font color='darkorange'>helix VII</font> . | |
| - | + | P2Y12R has some distinctive features from other GPCR structures in its family. <font color='lime'>Helix V</font>, for example, has around two more helical turns and does not have the typical helical bend that other GPCR structures have. As mentioned above, <font color='lime'>helix V</font> is <scene name='48/483887/Helix_vii/1'>elongated and straightened</scene> because the structure lacks proline and glycine residues to destabilize its structure. Furthermore, the elongated and straightened conformation causes P2Y12R’s extracellular end to shift 6 Å closer to <font color='turquoise'>helix IV</font> compared to other class A GPCR structures. In addition, the intracellular tip of <font color='darkorange'>helix VII</font> is closer to the seven transmembrane helical bundle. <font color='gold'>Helix VI’s</font> intracellular tip is tilted slightly outward and shifted closer to the intracellular surface than other GPCR structures. | |
| - | + | ||
| - | + | This <scene name='48/483887/Polar__nonpolar/1'>view</scene> demonstrates the polar and nonpolar regions of the P2Y12R's structure. AZD1283 spans more than 17 Å between <font color='turquoise'>helix IV</font> and <font color='darkorange'>helix VII</font> contributing to the polar and hydrophobic bonding with helices III–VI. | |
| - | + | ||
| - | + | ||
| - | + | ||
| Line 46: | Line 42: | ||
The P2Y12 receptor is mainly found on the surface of blood platelets, and functions as a regulator in platelet activation and blood clotting. The P2Y12 receptor is a G-protein coupled receptor, <scene name='48/483887/Ss/1'>a seven-transmembrane domain protein</scene> (characterized by <font color='pink'>seven alpha helices each</font>) linked to the cAMP-signaling pathway. It mediates platelet activation by decreasing intracellular cAMP levels through inhibition of an AC-mediated signaling pathway.[1] Acting as a chemoreceptor, P2Y12 utilizes ADP as an agonist, which initiates ADP-induced platelet aggregation. Clopidogrel in covalent binding complex with P2Y12 acts as an anti platelet agent, specifically as an ADP receptor inhibitor to decrease platelet aggregation and inhibit thrombus formation.[3] | The P2Y12 receptor is mainly found on the surface of blood platelets, and functions as a regulator in platelet activation and blood clotting. The P2Y12 receptor is a G-protein coupled receptor, <scene name='48/483887/Ss/1'>a seven-transmembrane domain protein</scene> (characterized by <font color='pink'>seven alpha helices each</font>) linked to the cAMP-signaling pathway. It mediates platelet activation by decreasing intracellular cAMP levels through inhibition of an AC-mediated signaling pathway.[1] Acting as a chemoreceptor, P2Y12 utilizes ADP as an agonist, which initiates ADP-induced platelet aggregation. Clopidogrel in covalent binding complex with P2Y12 acts as an anti platelet agent, specifically as an ADP receptor inhibitor to decrease platelet aggregation and inhibit thrombus formation.[3] | ||
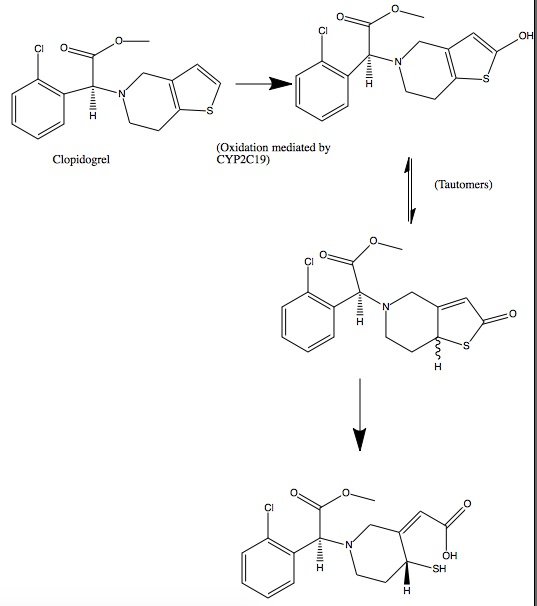
| - | Clopidogrel is a pro-drug and thienopyridine-type inhibitor of the P2Y12 receptor, which requires Cytochrome P450 to hepatically transform it to exert its anti platelet effect.[4] <scene name='48/483887/Aa/1'>Cytochrome P450</scene> is a membrane protein, characteristic of alternating <font color='gray'>hydrophobic</font> and <font color='pink'>polar</font> groups. The central heme group is stabilized by several side chains. The <font color='orange'>Fe2+</font> atom that makes up the heart of the heme group is surrounded by a <font color='blue'>highly hydrophobic porphyrin ring</font> . The role of the heme group in biological systems is to facilitate oxygen transport as well as aid in electron transfer as part of the electron transport chain. The heme group of the hemeprotein Cytochrome P450 acts as a catalyst for the metabolism of clopidogrel.[2] Shown below is the activation of Clopidogrel in vivo. CYP2C19 is an enzymatic member of the cytochrome P450 mixed-function oxidase system. The final step is a hydrolysis to yield the active metabolite. | + | Clopidogrel is a pro-drug and thienopyridine-type inhibitor of the P2Y12 receptor, which requires Cytochrome P450 to hepatically transform it to exert its anti platelet effect.[4] <scene name='48/483887/Aa/1'>Cytochrome P450</scene> is a membrane protein, characteristic of alternating <font color='gray'>hydrophobic</font> and <font color='pink'>polar</font> groups. The central heme group is stabilized by several side chains. The <font color='orange'>Fe2+</font> atom that makes up the heart of the heme group is surrounded by a <font color='blue'>highly hydrophobic porphyrin ring</font>. The role of the heme group in biological systems is to facilitate oxygen transport as well as aid in electron transfer as part of the electron transport chain. The heme group of the hemeprotein Cytochrome P450 acts as a catalyst for the metabolism of clopidogrel.[2] Shown below is the activation of Clopidogrel in vivo. CYP2C19 is an enzymatic member of the cytochrome P450 mixed-function oxidase system. The final step is a hydrolysis to yield the active metabolite. |
[[Image:Clop converted.jpg]] | [[Image:Clop converted.jpg]] | ||
Current revision
| This Sandbox is Reserved from January 19, 2016, through August 31, 2016 for use for Proteopedia Team Projects by the class Chemistry 423 Biochemistry for Chemists taught by Lynmarie K Thompson at University of Massachusetts Amherst, USA. This reservation includes Sandbox Reserved 425 through Sandbox Reserved 439. |
P2Y12 Receptor in Complex with AZD1283 (4ntj)[1]
by [Cora Ricker, Lauren Timmins, Aidan Finnerty, Adam Murphy, Duy Nguyen]
Student Projects for UMass Chemistry 423 Spring 2016
| |||||||||||