Sandbox Reserved 1177
From Proteopedia
(Difference between revisions)
| (3 intermediate revisions not shown.) | |||
| Line 4: | Line 4: | ||
==Introduction== | ==Introduction== | ||
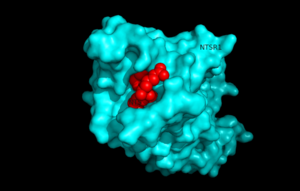
[[Image:surfaceprotein.png |300 px|below|thumb|'''Figure 1'''.Top view of NTSR1 protein interacting with NTS ligand]] | [[Image:surfaceprotein.png |300 px|below|thumb|'''Figure 1'''.Top view of NTSR1 protein interacting with NTS ligand]] | ||
| - | Neurotensin receptor 1 (NTSR1) is a '''[https://en.wikipedia.org/wiki/G_protein%E2%80%93coupled_receptor G-protein coupled receptor (GPCR).]''' GPCRs are a class of proteins with an extracellular binding domain and 7 transmembrane helices found only in '''[https://en.wikipedia.org/wiki/Eukaryote. eukaryotes]''' that assist in propagating a cellular response. This is accomplished by the binding of molecules to the GPCR outside the cell,causing a conformational change and activating a signal transduction pathway via '''[https://en.wikipedia.org/wiki/Second_messenger_system second messengers]''' such as '''[https://en.wikipedia.org/wiki/Cyclic_adenosine_monophosphate. cyclic AMP]''', '''[https://en.wikipedia.org/wiki/Inositol_trisphosphate. inositol triphosphate]''', and '''[https://en.wikipedia.org/wiki/Diglyceride . diacylglycerol]'''.<ref name="SPGP"/>. NTSR1 binds to the 13 amino acid peptide, neurotensin (NTS)<ref name="SONT">PMID:23051748</ref>, and the majority of the effects of NTS are mediated through NTSR1<ref name="SONT"/>. NTS has a variety of biological activities including a role in the leptin signalling pathways <ref name="Mice">PMID: 20211191</ref>, tumor growth <ref name="cancer">PMID:16887236</ref>, and dopamine regulation <ref name="Schizophrenia">PMID:22596253</ref>.Recently NTSR1 was crystallized bound with the C-terminus of its tridecapeptide ligand, <scene name='72/721548/Neurotensin/7'>NTS(8-13)</scene>. The shortened ligand was used because it has a higher potency and efficacy than its full-length counterpart<ref name="SONT"/>. Class A GPCRs bind their ligands within the transmembrane core in a ligand binding pocket. The <scene name='72/721547/Hydrophobic_binding_pocket/5'>ligand binding pocket</scene> in NTSR1 is located at the top of the protein (Figure 1). NTSR1 also contains an '''[https://en.wikipedia.org/wiki/Allosteric_regulation allosteric]''' <scene name='72/721548/Na_bind_pocket/13'>Na Binding Pocket</scene>. The Na+ binding pocket is located directly beneath the ligand binding pocket and the two pockets are separated by the residue W321. <ref name="SPGP">PMID:26205105</ref>. NTSR1 has been mutated to exist in both <scene name='72/721548/Ntsr1-elf/ | + | Neurotensin receptor 1 (NTSR1) is a '''[https://en.wikipedia.org/wiki/G_protein%E2%80%93coupled_receptor G-protein coupled receptor (GPCR).]''' GPCRs are a class of proteins with an extracellular binding domain and 7 transmembrane helices found only in '''[https://en.wikipedia.org/wiki/Eukaryote. eukaryotes]''' that assist in propagating a cellular response. This is accomplished by the binding of molecules to the GPCR outside the cell,causing a conformational change and activating a signal transduction pathway via '''[https://en.wikipedia.org/wiki/Second_messenger_system second messengers]''' such as '''[https://en.wikipedia.org/wiki/Cyclic_adenosine_monophosphate. cyclic AMP]''', '''[https://en.wikipedia.org/wiki/Inositol_trisphosphate. inositol triphosphate]''', and '''[https://en.wikipedia.org/wiki/Diglyceride . diacylglycerol]'''.<ref name="SPGP"/>. NTSR1 binds to the 13 amino acid peptide, neurotensin (NTS)<ref name="SONT">PMID:23051748</ref>, and the majority of the effects of NTS are mediated through NTSR1<ref name="SONT"/>. NTS has a variety of biological activities including a role in the leptin signalling pathways <ref name="Mice">PMID: 20211191</ref>, tumor growth <ref name="cancer">PMID:16887236</ref>, and dopamine regulation <ref name="Schizophrenia">PMID:22596253</ref>.Recently NTSR1 was crystallized bound with the C-terminus of its tridecapeptide ligand, <scene name='72/721548/Neurotensin/7'>NTS(8-13)</scene>. The shortened ligand was used because it has a higher potency and efficacy than its full-length counterpart<ref name="SONT"/>. Class A GPCRs bind their ligands within the transmembrane core in a ligand binding pocket. The <scene name='72/721547/Hydrophobic_binding_pocket/5'>ligand binding pocket</scene> in NTSR1 is located at the top of the protein (Figure 1). NTSR1 also contains an '''[https://en.wikipedia.org/wiki/Allosteric_regulation allosteric]''' <scene name='72/721548/Na_bind_pocket/13'>Na Binding Pocket</scene>. The Na+ binding pocket is located directly beneath the ligand binding pocket and the two pockets are separated by the residue W321. <ref name="SPGP">PMID:26205105</ref>. NTSR1 has been mutated to exist in both <scene name='72/721548/Ntsr1-elf/6'>active</scene>and <scene name='72/721547/Ntsr1-gw5/8'>active-like</scene> states. This has led to a greater understanding of the structure of NTSR1 and how the structure influences its function. |
== Structure == | == Structure == | ||
| Line 10: | Line 10: | ||
On the extracellular side of the protein is the | On the extracellular side of the protein is the | ||
<scene name='72/721547/Hydrophobic_binding_pocket/5'>hydrophobic binding pocket</scene>. | <scene name='72/721547/Hydrophobic_binding_pocket/5'>hydrophobic binding pocket</scene>. | ||
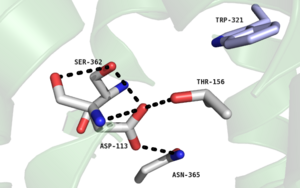
| - | One key residue in this pocket is a Phenylalanine at position 358 | + | One key residue in this pocket is a Phenylalanine at position 358, and its purpose is to take part in a network of hydrophobic stacking interactions. These interactions stabilize the Trp321 and Tyr324 residues allowing Tyr324 to interact with the C-terminal |
<scene name='72/721547/Ligand_protein_interactions/7'>Leu13 residue of the NTS ligand</scene> | <scene name='72/721547/Ligand_protein_interactions/7'>Leu13 residue of the NTS ligand</scene> | ||
via '''[https://en.wikipedia.org/wiki/Van_der_Waals_force Van der Waals interactions]''' . | via '''[https://en.wikipedia.org/wiki/Van_der_Waals_force Van der Waals interactions]''' . | ||
| Line 28: | Line 28: | ||
===Active State=== | ===Active State=== | ||
| - | After determining <scene name='72/721547/Ntsr1-gw5/ | + | After determining <scene name='72/721547/Ntsr1-gw5/8'>NTSR1-GW5</scene> as only active-like, research was conducted to determine the structure of NTSR1 with catalytic nucleotide exchange. In order to do so, three of the six mutations were reverted back <ref name="SPGP"/>, and these three residues were selected on the basis of their location. The reversion of E166A, L310A, and F358A led to NTSR1 with G-protein activity at almost wild-type level. This protein was named, <scene name='72/721548/Ntsr1-elf/6'>NTSR1-ELF</scene>, and indicated that the amino acid residues E166, L310, and F358 play significant roles in the activity of NTSR1 <ref name="SPGP"/>. |
====Leu310==== | ====Leu310==== | ||
Current revision
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 Krumm BE, White JF, Shah P, Grisshammer R. Structural prerequisites for G-protein activation by the neurotensin receptor. Nat Commun. 2015 Jul 24;6:7895. doi: 10.1038/ncomms8895. PMID:26205105 doi:http://dx.doi.org/10.1038/ncomms8895
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 White JF, Noinaj N, Shibata Y, Love J, Kloss B, Xu F, Gvozdenovic-Jeremic J, Shah P, Shiloach J, Tate CG, Grisshammer R. Structure of the agonist-bound neurotensin receptor. Nature. 2012 Oct 25;490(7421):508-13. doi: 10.1038/nature11558. Epub 2012 Oct 10. PMID:23051748 doi:http://dx.doi.org/10.1038/nature11558
- ↑ 3.0 3.1 3.2 3.3 Liang Y, Boules M, Li Z, Williams K, Miura T, Oliveros A, Richelson E. Hyperactivity of the dopaminergic system in NTS1 and NTS2 null mice. Neuropharmacology. 2010 Jun;58(8):1199-205. doi:, 10.1016/j.neuropharm.2010.02.015. Epub 2010 Mar 6. PMID:20211191 doi:http://dx.doi.org/10.1016/j.neuropharm.2010.02.015
- ↑ 4.0 4.1 4.2 Carraway RE, Plona AM. Involvement of neurotensin in cancer growth: evidence, mechanisms and development of diagnostic tools. Peptides. 2006 Oct;27(10):2445-60. Epub 2006 Aug 2. PMID:16887236 doi:http://dx.doi.org/10.1016/j.peptides.2006.04.030
- ↑ 5.0 5.1 5.2 Griebel G, Holsboer F. Neuropeptide receptor ligands as drugs for psychiatric diseases: the end of the beginning? Nat Rev Drug Discov. 2012 May 18;11(6):462-78. doi: 10.1038/nrd3702. PMID:22596253 doi:http://dx.doi.org/10.1038/nrd3702