Sandbox Reserved 1176
From Proteopedia
(Difference between revisions)
| (83 intermediate revisions not shown.) | |||
| Line 3: | Line 3: | ||
==Introduction== | ==Introduction== | ||
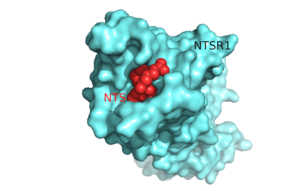
| - | [[Image: | + | [[Image:Surface_Protein_red.png |300 px|below|thumb|'''Figure 1'''.Top view of NTSR1 protein (blue) interacting with its ligand, NTS(red).]] |
| - | Neurotensin receptor 1 (NTSR1) is a '''[https://en.wikipedia.org/wiki/G_protein%E2%80%93coupled_receptor G-protein coupled receptor (GPCR).]''' GPCRs are a class of proteins with an extracellular binding domain and 7 transmembrane helices | + | Neurotensin receptor 1 (NTSR1) is a '''[https://en.wikipedia.org/wiki/G_protein%E2%80%93coupled_receptor G-protein coupled receptor (GPCR).]''' GPCRs are a class of proteins with an extracellular binding domain and 7 transmembrane helices that assist in propagating a cellular response<ref name="SPGP"/>. This is accomplished by the binding of ligands to the GPCR outside the cell, causing a '''[https://en.wikipedia.org/wiki/Conformational_change conformational change]''' and activating a '''[https://en.wikipedia.org/wiki/Signal_transduction signal transduction]''' pathway via '''[https://en.wikipedia.org/wiki/Second_messenger_system second messengers]''' such as '''[https://en.wikipedia.org/wiki/Cyclic_adenosine_monophosphate cyclic AMP]''', '''[https://en.wikipedia.org/wiki/Inositol_trisphosphate inositol triphosphate]''', and '''[https://en.wikipedia.org/wiki/Diglyceride diacylglycerol]'''.<ref name="SPGP"/> The ligand for NTSR1 is the 13 amino acid peptide, neurotensin (NTS)<ref name="SONT">PMID:23051748</ref>, and the majority of the effects of NTS are mediated through NTSR1<ref name="SONT"/>. NTS has a variety of biological activities including a role in the '''[https://en.wikipedia.org/wiki/Leptin leptin]''' signaling pathways <ref name="Mice">PMID: 20211191</ref>, tumor growth <ref name="cancer">PMID:16887236</ref>, and '''[https://en.wikipedia.org/wiki/Dopamine dopamine]''' regulation <ref name="Schizophrenia">PMID:22596253</ref>. Recently NTSR1 was crystallized bound with the C-terminus of its tridecapeptide '''[https://en.wikipedia.org/wiki/Ligand ligand]''', <scene name='72/721548/Neurotensin/7'>NTS(8-13)</scene>. The shortened ligand was used because it has a higher potency and efficacy than its full-length counterpart<ref name="SONT"/>. Class A GPCRs bind their ligands within the '''[https://en.wikipedia.org/wiki/Transmembrane_protein transmembrane]''' core in a ligand binding pocket. The <scene name='72/721547/Hydrophobic_binding_pocket/6'>hydrophobic binding pocket</scene> in NTSR1 is located at the top of the protein (Figure 1). NTSR1 also contains an '''[https://en.wikipedia.org/wiki/Allosteric_regulation allosteric]''' <scene name='72/721548/Na_bind_pocket/13'>sodium binding pocket</scene>, which is located directly beneath the ligand binding pocket and the two pockets are separated by the residue <scene name='72/721548/Trp321/1'>Trp321</scene><ref name="SPGP">PMID:26205105</ref>. NTSR1 has been mutated to exist in both <scene name='72/721548/Ntsr1-elf/6'>active</scene> and <scene name='72/721547/Ntsr1-gw5/8'>active-like</scene> states. This has led to a greater understanding of the structure of NTSR1 and how the structure influences its function. |
== Structure == | == Structure == | ||
===Ligand Binding Pocket=== | ===Ligand Binding Pocket=== | ||
On the extracellular side of the protein is the | On the extracellular side of the protein is the | ||
| - | <scene name='72/721547/Hydrophobic_binding_pocket/ | + | <scene name='72/721547/Hydrophobic_binding_pocket/6'>hydrophobic binding pocket</scene>. <ref name="SONT"/> |
| - | One key residue in this pocket is a Phenylalanine at position 358, which takes part in a network of hydrophobic stacking interactions. These interactions stabilize the Trp321 and Tyr324 residues allowing Tyr324 to interact with the '''[https://en.wikipedia.org/wiki/C-terminus C-terminal]''' | + | One key residue in this pocket is a Phenylalanine at position 358, which takes part in a network of hydrophobic stacking interactions<ref name="SPGP"/>. These interactions stabilize the Trp321 and Tyr324 residues allowing Tyr324 to interact with the '''[https://en.wikipedia.org/wiki/C-terminus C-terminal]''' |
| - | <scene name='72/721547/Ligand_protein_interactions/ | + | <scene name='72/721547/Ligand_protein_interactions/8'>Leu13 residue of NTS ligand</scene> |
| - | via '''[https://en.wikipedia.org/wiki/Van_der_Waals_force Van der Waals interactions]''' . | + | via '''[https://en.wikipedia.org/wiki/Van_der_Waals_force Van der Waals interactions]''' .<ref name="SONT"/><ref name="SPGP"/> |
| - | Without the hydrophobic stacking interactions that are facilitated by the Phe358, this binding interaction would be destabilized. Trp321 also participates in these stacking interactions and serves as the boundary between the ligand binding pocket and the | + | Without the hydrophobic stacking interactions that are facilitated by the Phe358, this binding interaction would be destabilized. Trp321 also participates in these stacking interactions and serves as the boundary between the ligand binding pocket and the Na<sup>+</sup> binding pocket.<ref name="SPGP"/> |
===Na<sup>+</sup> Binding Pocket=== | ===Na<sup>+</sup> Binding Pocket=== | ||
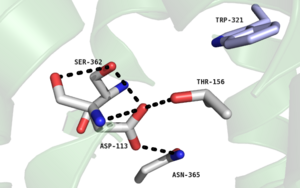
| - | [[Image:Na+ Proteopedia Picture.png |300 px|left|thumb|Figure 2. Residues of collapsed sodium binding pocket. Trp321 (blue) sets the top of the pocket, where Ser362, Asp113, Thr156 and Asn365 (gray) are involved in hydrogen bonding interactions preventing the coordination of a Na<sup>+</sup> ion.]] | + | [[Image:Na+ Proteopedia Picture.png |300 px|left|thumb|Figure 2. Residues of the collapsed sodium binding pocket. Trp321 (blue) sets the top of the pocket, where Ser362, Asp113, Thr156 and Asn365 (gray) are involved in hydrogen bonding interactions preventing the coordination of a Na<sup>+</sup> ion.]] |
| - | <scene name='72/721548/Trp321/1'>Trp321</scene>, which is positioned at the bottom of the <scene name='72/721547/Hydrophobic_binding_pocket/5'>hydrophobic binding pocket</scene>, sets the top of the <scene name='72/721548/Na_bind_pocket/12'> | + | <scene name='72/721548/Trp321/1'>Trp321</scene>, which is positioned at the bottom of the <scene name='72/721547/Hydrophobic_binding_pocket/5'>hydrophobic binding pocket</scene>, sets the top of the <scene name='72/721548/Na_bind_pocket/12'> sodium binding pocket</scene>. The Na<sup>+</sup> ion binding pocket acts as a negative allosteric site for G protein activity <ref name="SPGP"/>. When Na<sup>+</sup> enters the Na<sup>+</sup> ion binding pocket, it coordinates with Asp95, Gln131, Ser135, and Asp113, decreasing the signaling activity of NTSR1 <ref name="SPGP"/>. When NTSR1 is in its active state, the Na<sup>+</sup> ion binding pocket is collapsed. This prevents the regulation of protein activity through a Na<sup>+</sup> ion, as the Na<sup>+</sup> ion is unable to coordinate via a salt bridge to Asp113 (Figure 2). The side chain atoms of Asp113 form a '''[https://en.wikipedia.org/wiki/Hydrogen_bond hydrogen bond]''' network with Thr156, Ser361, Ser362, and Gln365 instead, which prevents the coordination of a Na<sup>+</sup> ion<ref name ="SPGP"/> (Figure 2). |
==Activation of NTSR1== | ==Activation of NTSR1== | ||
| - | Since wild type NTSR1 was unstable in detergent solution, six residues in the protein were mutated for stabilization. | + | Since wild type NTSR1 was unstable in detergent solution for imaging, six residues in the protein were mutated for stabilization.<ref name="SONT"/> <ref name="SPGP"/> |
===Active-Like State=== | ===Active-Like State=== | ||
| - | The | + | The six amino acid mutations for thermostabilization <ref name="SONT"/> were Ala86Leu, Glu166Ala, Gly215Ala, Leu310Ala, Phe358Ala, and Val360Ala. This protein was found to have NTS affinity similar to that of wild tpye NTSR1, and was named <scene name='72/721547/Ntsr1-gw5/8'>NTSR1-GW5</scene>. Along with this, the Na+ ion binding pocket was collapsed in this protein. However, NTSR1-GW5 did not have G-protein activity <ref name="SONT"/>. |
===Active State=== | ===Active State=== | ||
| - | After determining <scene name='72/721547/Ntsr1-gw5/8'>NTSR1-GW5</scene> as only active-like, | + | After determining the original structural state, <scene name='72/721547/Ntsr1-gw5/8'>NTSR1-GW5</scene>, as only active-like, the structure of NTSR1 was determined in an active state.<ref name="SPGP"/> By reverting back three of the original six mutations from the active-like structure on the basis of their location<ref name="SPGP"/>, NTSR1 gained near wild-type activity.<ref name="SPGP"/> The three reversions were Asp166, Leu310, and Phe358, and this protein was named <scene name='72/721548/Ntsr1-elf/6'>NTSR1-ELF</scene>. The revival of activity in NTSR1 indicated that the reverted amino acid residues (Asp166, Leu310, and Phe358) play significant roles in G-protein activity.<ref name="SPGP"/> |
====Leu310==== | ====Leu310==== | ||
| - | <scene name='72/721548/L310/ | + | <scene name='72/721548/L310/6'>Leu310</scene> is crucial for interactions with the G alpha subunit by positioning Arg167 in the conserved <scene name='72/721548/Dery_motif/2'>D/ERY motif</scene><ref name="SPGP"/>. When Leu310 was substituted with alanine, Arg167 was able to form a stabilizing hydrogen bonding network with Asn257, Ser164 and Gly306, which oriented Arg167 in a position that was unfavorable for contacting the G alpha subunit. When residue 310 was converted back to leucine, this hydrogen bonding network was sterically unfavorable and Arg167 interacted with the G alpha subunit<ref name="SPGP"/> leading to the transduction of several different signals involved in dopamine regulation<ref name="Schizophrenia"/>, leptin signlaing<ref name="Mice"/>, and tumor growth<ref name="cancer"/>. |
| - | + | ||
====Phe358==== | ====Phe358==== | ||
| - | When this residue was mutated to an alanine <ref name="SPGP"/> the <scene name='72/721547/Hydrophobic_binding_pocket/ | + | When this residue was mutated to an alanine <ref name="SPGP"/> the <scene name='72/721547/Hydrophobic_binding_pocket/6'>hydrophobic stacking interactions</scene> of the ligand binding pocket were interrupted,resulting in a lack of G-Protein activity in NTSR1.<ref name="SPGP"/>.This supported the role of Phe358 as stated in the hydrophobic binding pocket section of this page. |
====Glu166==== | ====Glu166==== | ||
| - | Although the role of <scene name='72/721548/E166/ | + | Although the role of <scene name='72/721548/E166/4'>Glu166</scene> in G-protein activity is not quite as clear as it is for <scene name='72/721548/L310/3'>Leu310</scene> or <scene name='72/721547/Hydrophobic_binding_pocket/5'>Phe358</scene>, substituting this residue for an alanine significantly reduced '''[https://en.wikipedia.org/wiki/Active_site catalytic]''' G-protein activity <ref name="SPGP"/>. Glu166 is part of a <scene name='72/721548/Dery_motif/2'>D/ERY motif</scene> that is highly conserved in class A GPCRs and includes Arg167 and Tyr168. It's hypothesized <ref name="SPGP"/> that Glu166 interacts with Val102, Thr101,and His105 to stabilize the G protein. An important connection between the D/ERY motif and intracellular loop 2 via M181, has also been hypothesized.<ref name="SPGP"/>. ICL2 plays a role in the dissociation of the receptor-G protein complex with GTP present.<ref name="SPGP"/> |
== Biological Relevance == | == Biological Relevance == | ||
===Neurotensin=== | ===Neurotensin=== | ||
| - | <scene name='72/721548/Neurotensin/3'>Neurotensin</scene> is a 13 amino acid '''[https://en.wikipedia.org/wiki/Peptide peptide]''' that is found in both '''[https://en.wikipedia.org/wiki/Nervous_tissue nervous]''' and peripheral tissues. It functions as a '''[https://en.wikipedia.org/wiki/Hormone hormone]''' and a '''[https://en.wikipedia.org/wiki/Neurotransmitter neurotransmitter]''' by activating the G-protein coupled receptor NTSR1<ref name= "SPGP"/> | + | <scene name='72/721548/Neurotensin/3'>Neurotensin</scene> is a 13 amino acid '''[https://en.wikipedia.org/wiki/Peptide peptide]''' that is found in both '''[https://en.wikipedia.org/wiki/Nervous_tissue nervous]''' and peripheral tissues <ref name= "SPGP"/>. It functions as a '''[https://en.wikipedia.org/wiki/Hormone hormone]''' and a '''[https://en.wikipedia.org/wiki/Neurotransmitter neurotransmitter]''' by activating the G-protein coupled receptor, NTSR1<ref name= "SPGP"/>. |
===Leptin Research=== | ===Leptin Research=== | ||
| - | NTSR1 deficient mice were not able to receive a '''[https://en.wikipedia.org/wiki/Hunger_(motivational_state) satiety]''' signal.<ref name="Mice"/>The mice continued to eat when food was present, leading to significant weight gain. | + | NTSR1 deficient mice were not able to receive a '''[https://en.wikipedia.org/wiki/Hunger_(motivational_state) satiety]''' signal from '''[https://en.wikipedia.org/wiki/Leptin Leptin]'''<ref name="Mice"/>. The mice continued to eat when food was present, leading to significant weight gain. With an NTSR1 deficiency, NTS does not bind efficiently to NTSR1, and the leptin signaling pathway is interrupted <ref name="Mice"/>. |
===Cancer Studies=== | ===Cancer Studies=== | ||
| - | Some tumor cells can secrete and express | + | Some tumor cells can secrete and express NTS and NTS receptors themselves suggesting that NTS '''[https://en.wikipedia.org/wiki/Autocrine_signalling autocrine]''', '''[https://en.wikipedia.org/wiki/Endocrine_system endocrine]''' and '''[https://en.wikipedia.org/wiki/Paracrine_signalling paracrine]''' regulation are possible. This leads to aggressive growth and tumor development. Injecting animals with NTS increased tumor growth and size, while injecting them with NTS antagonist had the opposite effect <ref name="cancer"/>. NTS regulation may be used in future cancer treatments. |
===Dopamine Regulation=== | ===Dopamine Regulation=== | ||
| - | The '''[http://www.schizophreniaforum.org/for/curr/AbiDargham/ dopamine hypothesis]''' states that hyperdopamine levels may lead to schizophrenic symptoms. NTSR1 | + | The '''[http://www.schizophreniaforum.org/for/curr/AbiDargham/ dopamine hypothesis]''' states that hyperdopamine levels may lead to schizophrenic symptoms. NTSR1 causes a blockade which inhibits firing in dopaminergic cells suggesting that NTSR1 could be used in schizophrenia treatment. However, this led to extreme secondary effects and was discontinued. Despite this, research on NTSR1 as a treatment for schizophrenia persists<ref name="Schizophrenia"/>. |
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 Krumm BE, White JF, Shah P, Grisshammer R. Structural prerequisites for G-protein activation by the neurotensin receptor. Nat Commun. 2015 Jul 24;6:7895. doi: 10.1038/ncomms8895. PMID:26205105 doi:http://dx.doi.org/10.1038/ncomms8895
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 White JF, Noinaj N, Shibata Y, Love J, Kloss B, Xu F, Gvozdenovic-Jeremic J, Shah P, Shiloach J, Tate CG, Grisshammer R. Structure of the agonist-bound neurotensin receptor. Nature. 2012 Oct 25;490(7421):508-13. doi: 10.1038/nature11558. Epub 2012 Oct 10. PMID:23051748 doi:http://dx.doi.org/10.1038/nature11558
- ↑ 3.0 3.1 3.2 3.3 Liang Y, Boules M, Li Z, Williams K, Miura T, Oliveros A, Richelson E. Hyperactivity of the dopaminergic system in NTS1 and NTS2 null mice. Neuropharmacology. 2010 Jun;58(8):1199-205. doi:, 10.1016/j.neuropharm.2010.02.015. Epub 2010 Mar 6. PMID:20211191 doi:http://dx.doi.org/10.1016/j.neuropharm.2010.02.015
- ↑ 4.0 4.1 4.2 Carraway RE, Plona AM. Involvement of neurotensin in cancer growth: evidence, mechanisms and development of diagnostic tools. Peptides. 2006 Oct;27(10):2445-60. Epub 2006 Aug 2. PMID:16887236 doi:http://dx.doi.org/10.1016/j.peptides.2006.04.030
- ↑ 5.0 5.1 5.2 Griebel G, Holsboer F. Neuropeptide receptor ligands as drugs for psychiatric diseases: the end of the beginning? Nat Rev Drug Discov. 2012 May 18;11(6):462-78. doi: 10.1038/nrd3702. PMID:22596253 doi:http://dx.doi.org/10.1038/nrd3702