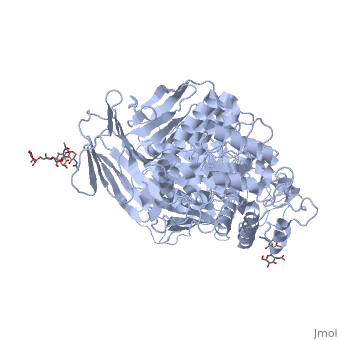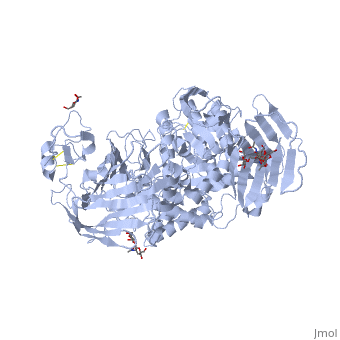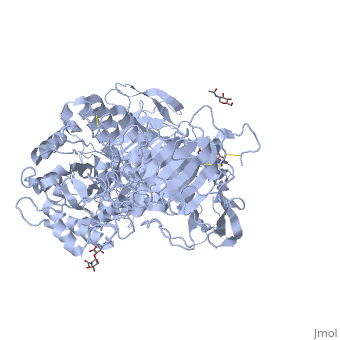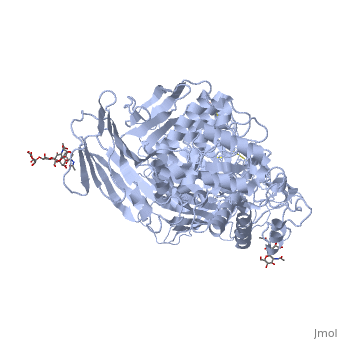
Sucrase-isomaltase
From Proteopedia
(Difference between revisions)
(New page: <Structure load='3lpo' size='350' frame='true' align='right' caption='Four isomaltase complexes' scene='Insert optional scene name here' /> == Function == Sucrase isomaltase is a partia...) |
|||
| (8 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | < | + | <StructureSection load='3lpo' size='350' side='right' scene='' caption='Human glycosylated sucrase-isomaltase N-terminal domain (PDB code [[3lpo]])'> |
| - | + | ||
== Function == | == Function == | ||
| - | Sucrase isomaltase is a partially embedded integral protein located in the brush border of the small intestine. SI is responsible for catalyzing the hydrolysis of dietary carbohydrates that includes starch, sucrose, and isomaltase. This hydrolysis leads to ATP production after further processing. | + | '''Sucrase isomaltase''' (SI) is a partially embedded integral protein located in the brush border of the small intestine. SI is responsible for catalyzing the hydrolysis of dietary carbohydrates that includes starch, sucrose, and isomaltase. This hydrolysis leads to ATP production after further processing. |
The brush border is important for the functional absorption of minerals and amino acids so the body can properly use them. The border is composed of millions of microvilli tightly packed together that are anywhere from 100 to 2,000 nanometers. Absorption is effective at a location such as this due to the large surface area being open for contact. <ref> http://www.siumed.edu/~dking2/erg/gicells.htm</ref> | The brush border is important for the functional absorption of minerals and amino acids so the body can properly use them. The border is composed of millions of microvilli tightly packed together that are anywhere from 100 to 2,000 nanometers. Absorption is effective at a location such as this due to the large surface area being open for contact. <ref> http://www.siumed.edu/~dking2/erg/gicells.htm</ref> | ||
Some other key enzymes that capitalize on this characteristic in the small intestine are: glucoamylase (Maltase), lactase, and peptidases. Among these common enzymes however is SI, which is important because it is critically involved in the final stage of carbohydrate digestion. <ref> http://www.uniprot.org/uniprot/P14410</ref> Specifically, this enzyme works by catalyzing the conversion of isomaltose into glucose molecules. Cleaving bonds with water (hydrolysis), SI interacts with glucosidic linkages produced by alpha-amylase. <ref>Maureen Barlow Pugh, ed. (2000). Stedman's Medical Dictionary (27th ed.). Baltimore, Maryland, USA: Lippincott Williams & Wilkins. p. 65. ISBN 978-0-683-40007-6. </ref> | Some other key enzymes that capitalize on this characteristic in the small intestine are: glucoamylase (Maltase), lactase, and peptidases. Among these common enzymes however is SI, which is important because it is critically involved in the final stage of carbohydrate digestion. <ref> http://www.uniprot.org/uniprot/P14410</ref> Specifically, this enzyme works by catalyzing the conversion of isomaltose into glucose molecules. Cleaving bonds with water (hydrolysis), SI interacts with glucosidic linkages produced by alpha-amylase. <ref>Maureen Barlow Pugh, ed. (2000). Stedman's Medical Dictionary (27th ed.). Baltimore, Maryland, USA: Lippincott Williams & Wilkins. p. 65. ISBN 978-0-683-40007-6. </ref> | ||
| + | See [[Alpha-glucosidase]] | ||
== Structure == | == Structure == | ||
| Line 45: | Line 45: | ||
-The gene on which SI processes occur is located on chromosome 3 and is composed of 48 exons. | -The gene on which SI processes occur is located on chromosome 3 and is composed of 48 exons. | ||
| + | </StructureSection> | ||
== References == | == References == | ||
<references /> | <references /> | ||
| + | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
References
- ↑ http://www.siumed.edu/~dking2/erg/gicells.htm
- ↑ http://www.uniprot.org/uniprot/P14410
- ↑ Maureen Barlow Pugh, ed. (2000). Stedman's Medical Dictionary (27th ed.). Baltimore, Maryland, USA: Lippincott Williams & Wilkins. p. 65. ISBN 978-0-683-40007-6.
- ↑ "SI sucrase-isomaltase (alpha-glucosidase) [Homo sapiens (human)] - Gene - NCBI"
- ↑ Naim HY, Sterchi EE, Lentze MJ (1988). "Biosynthesis of the human sucrase-isomaltase complex. Differential O-glycosylation of the sucrase subunit correlates with its position within the enzyme complex". J. Biol. Chem. 263 (15): 7242–53. PMID 3366777
- ↑ http://www.jbc.org/content/254/6/1821.full.pdf
- ↑ http://onlinelibrary.wiley.com/doi/10.1111/j.1432-1033.1977.tb11335.x/epdf
- ↑ http://www.jbc.org/content/251/11/3250.full.pdf
- ↑ http://www.iffgd.org/site/gi-disorders/other/csid
- ↑ Sim L, Willemsma C, Mohan S, Naim HY, Pinto BM, Rose DR (2010). "Structural basis for substrate selectivity in human maltase-glucoamylase and sucrase-isomaltase N-terminal domains". J. Biol. Chem. 285 (23): 17763–70. doi:10.1074/jbc.M109.078980. PMC 2878540. PMID 20356844