Human gastric lipase
From Proteopedia
(Difference between revisions)
| (9 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | < | + | <StructureSection load='' size='350' side='right' caption='Structure of Human Gastric Lipase (PDB input: [[1hlg]])' scene='72/728237/Overall/2'> |
== Introduction == | == Introduction == | ||
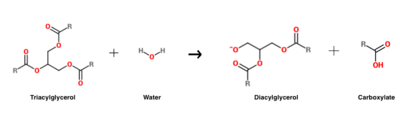
| - | Human gastric lipase (HGL, E.C. 3.1.1.3) (PBD ID: 1hlg) is the [[lipase]] that is responsible for initiating the digestion of dietary fats in the stomach <ref name="armand">PMID:7598069</ref>. This acid-stable enzyme <ref name="gastritis">PMID:23899880</ref> is secreted by the fundic chief cells of the human stomach and catalyzes 10-20% of total lipolytic processes (i.e., those involving fat breakdown) in healthy adults <ref name="armand" />. HGL specifically catalyzes the hydrolysis of triacylglycerol in order to produce diacylglycerol and a carboxylate byproduct <ref name="roussel">PMID:10358049</ref>, a process that facilitates subsequent fat breakdown by pancreatic lipase <ref name="dogs">PMID:20965171</ref>. In terms of disease implications, there is evidence to suggest that HGL secretion is altered in individuals with gastritis (the most common gastric condition, in which the stomach lining is inflamed) <ref name="gastritis" />. Furthermore, individuals with compromised pancreatic function (and therefore reduced levels of pancreatic lipase) must depend heavily on HGL in order to digest dietary fats <ref name="armand" />. | + | '''Human gastric lipase''' (HGL, E.C. 3.1.1.3) (PBD ID: [[1hlg]]) is the [[lipase]] that is responsible for initiating the digestion of dietary fats in the stomach <ref name="armand">PMID:7598069</ref>. This acid-stable enzyme <ref name="gastritis">PMID:23899880</ref> is secreted by the fundic chief cells of the human stomach and catalyzes 10-20% of total lipolytic processes (i.e., those involving fat breakdown) in healthy adults <ref name="armand" />. HGL specifically catalyzes the hydrolysis of triacylglycerol in order to produce diacylglycerol and a carboxylate byproduct <ref name="roussel">PMID:10358049</ref>, a process that facilitates subsequent fat breakdown by pancreatic lipase <ref name="dogs">PMID:20965171</ref>. In terms of disease implications, there is evidence to suggest that HGL secretion is altered in individuals with gastritis (the most common gastric condition, in which the stomach lining is inflamed) <ref name="gastritis" />. Furthermore, individuals with compromised pancreatic function (and therefore reduced levels of pancreatic lipase) must depend heavily on HGL in order to digest dietary fats <ref name="armand" />. |
== Structural Highlights == | == Structural Highlights == | ||
| - | HGL, a | + | HGL, a [[hydrolase]] enzyme consisting of two 379 amino acid residue-long subunits, possesses a <scene name='72/728060/Catalytic_elbow/3'>Catalytic Arm</scene> that contains residues Ser-153, His-353, and Asp-324. This structure is essential to the breakdown of lipids, coordinated with an <scene name='72/728060/Arm_and_hole/1'>Oxyanion Hole</scene> at Leu-67 and Gln-154 <ref name="dogs">PMID:20965171</ref>, that serves to stabilize the transition state. Structurally, the human gastric lipase exhibits a complex <scene name='72/728237/Overall/1'>Secondary Structure</scene> (beta sheets shown in yellow, alpha helices shown in orange, coiled coils shown in green, and resolved carbohydrates shown as purple). The <scene name='72/728237/1hlg_lid_and_catalytic_arm/4'>Lid</scene> of HGL at residues 215-244 <ref name="dogs">PMID:20965171</ref> gives way to the <scene name='72/728060/Hydrophobic_regions/1'>Hydrophobic Areas</scene> (hydrophobic regions noted in red) both surrounding the active site and interfacing the lid. These areas are thought to draw lipids and promote docking <ref name="roussel" />. |
== Function == | == Function == | ||
| Line 27: | Line 27: | ||
== External Links == | == External Links == | ||
| - | + | PDB - 1HLG: http://www.rcsb.org/pdb/explore.do?structureId=1HLG | |
| - | + | </StructureSection> | |
| - | PDB | + | |
| - | + | ||
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Armand M, Hamosh M, DiPalma JS, Gallagher J, Benjamin SB, Philpott JR, Lairon D, Hamosh P. Dietary fat modulates gastric lipase activity in healthy humans. Am J Clin Nutr. 1995 Jul;62(1):74-80. PMID:7598069
- ↑ 2.0 2.1 2.2 2.3 Tomasik PJ, Wedrychowicz A, Rogatko I, Zajac A, Fyderek K, Sztefko K. Gastric lipase secretion in children with gastritis. Nutrients. 2013 Jul 29;5(8):2924-32. doi: 10.3390/nu5082924. PMID:23899880 doi:http://dx.doi.org/10.3390/nu5082924
- ↑ 3.0 3.1 Roussel A, Canaan S, Egloff MP, Riviere M, Dupuis L, Verger R, Cambillau C. Crystal structure of human gastric lipase and model of lysosomal acid lipase, two lipolytic enzymes of medical interest. J Biol Chem. 1999 Jun 11;274(24):16995-7002. PMID:10358049
- ↑ 4.0 4.1 4.2 Selvan A, Seniya C, Chandrasekaran SN, Siddharth N, Anishetty S, Pennathur G. Molecular dynamics simulations of human and dog gastric lipases: insights into domain movements. FEBS Lett. 2010 Nov 19;584(22):4599-605. doi: 10.1016/j.febslet.2010.10.021. Epub, 2010 Oct 20. PMID:20965171 doi:http://dx.doi.org/10.1016/j.febslet.2010.10.021
- ↑ Adapted from [1]; image generated using [2]
- ↑ Gargouri Y, Pieroni G, Riviere C, Sauniere JF, Lowe PA, Sarda L, Verger R. Kinetic assay of human gastric lipase on short- and long-chain triacylglycerol emulsions. Gastroenterology. 1986 Oct;91(4):919-25. PMID:3743968
- ↑ Pfeffer JM, Weadge JT, Clarke AJ. Mechanism of action of Neisseria gonorrhoeae O-acetylpeptidoglycan esterase, an SGNH serine esterase. J Biol Chem. 2013 Jan 25;288(4):2605-13. doi: 10.1074/jbc.M112.436352. Epub 2012 , Dec 3. PMID:23209280 doi:http://dx.doi.org/10.1074/jbc.M112.436352
- ↑ Wojdemann M, Wettergren A, Sternby B, Holst JJ, Larsen S, Rehfeld JF, Olsen O. Inhibition of human gastric lipase secretion by glucagon-like peptide-1. Dig Dis Sci. 1998 Apr;43(4):799-805. PMID:9558037
- ↑ Wojdemann M, Riber C, Bisgaard T, Sternby B, Larsen S, Rehfeld JF, Holst JJ, Olsen O. Inhibition of human gastric lipase by intraduodenal fat involves glucagon-like peptide-1 and cholecystokinin. Regul Pept. 1999 Apr 30;80(3):101-6. PMID:10425652
Proteopedia Page Contributors and Editors (what is this?)
Megan M. Roy, Giavanna Verdi, Karsten Theis, Michal Harel, Alexander Berchansky, Aubrey A. Siebels