We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Dicer
From Proteopedia
(Difference between revisions)
| (13 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
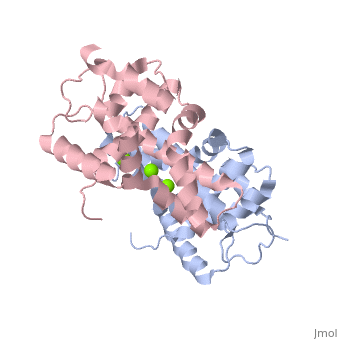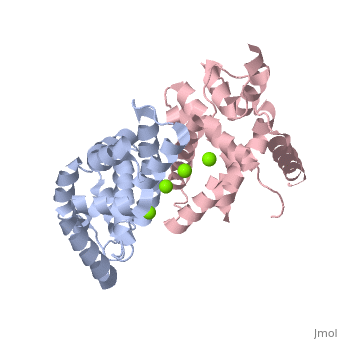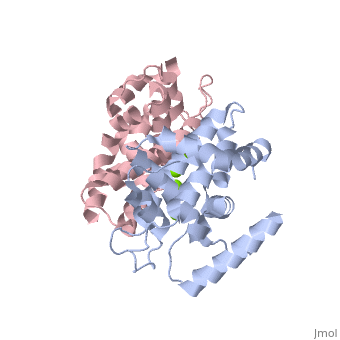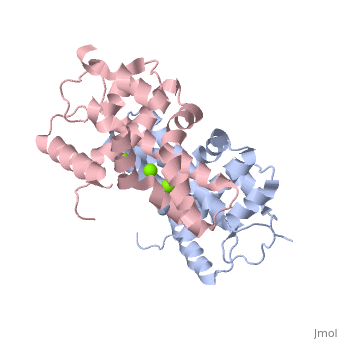
| - | + | <StructureSection load='2EB1' size='350' side='right' caption='Human Endoribonuclease Dicer C-terminal complex with Mg+2 ion (green) (PDB code [[2eb1]])' scene=''> | |
| - | <StructureSection load=' | + | |
==Introduction== | ==Introduction== | ||
| - | + | '''Dicer''', or '''endoribonuclease Dicer''', was discovered/named in 2001 by Emily Bernstein. She was a graduate student in Greg Hannon's lab at the Cold Spring Harbor Laboratory in New York. She was trying to discover the enzyme that was responsible for removing small RNA fragments from double-stranded RNA. The dicer enzyme was found by isolating it from the RISC complex in the RNAi mechanism. It was known that RISC was not responsible for chopping up these small RNA fragments, so this complex was isolated from the system to locate the enzyme that was the source for these RNA fragments.<ref>PMID: 11201747</ref> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Dicer is a type of [[Ribonuclease]] that processes potentially harmful double-stranded RNA (dsRNA) into microRNA and small-interfering RNA (siRNA) to be used in the process of RNA interference. Dicer is commonly utilized by cells in order to prevent the assimilation of viral DNA into the cells’ genome. The viral DNA is butchered into smaller segments that are each about 21 nucleotides long; the cut take places at the 5’ phosphate and the 3’ hydroxyl, and usually includes a 2 nucleotide overhang. There is a single processing center in HS Dicer implying that there are two catalytic sites which help form products with the 2 3' overhang. These newly formed segments attach themselves to single stranded mRNA which ultimately leads to mRNA degradation by the cell and translational suppression. The dicer enzyme in humans contains three domains: the <scene name='70/706244/Rnase_iii_1/1'>RNase III 1</scene>, <scene name='70/706244/Rnase_iii_2/1'>RNase III 2</scene>, and the <scene name='70/706244/Paz_domain/1'>Paz Domain</scene>.<ref name=jmol>PMID: 16410517</ref> There are three classes of RNase III proteins which are divided into categories called Escherichia coli RNase III, <scene name='70/706244/Drosha/1'>Drosha</scene>, and Dicer which are given the numbers one, two, and three respectively. The Escherichia coli RNase III class has one domain while the Drosha and dicer have two domains each. There is no evidence of the first class of enzymes in mammals.<ref name=biochem>PMID: 17920623</ref> | Dicer is a type of [[Ribonuclease]] that processes potentially harmful double-stranded RNA (dsRNA) into microRNA and small-interfering RNA (siRNA) to be used in the process of RNA interference. Dicer is commonly utilized by cells in order to prevent the assimilation of viral DNA into the cells’ genome. The viral DNA is butchered into smaller segments that are each about 21 nucleotides long; the cut take places at the 5’ phosphate and the 3’ hydroxyl, and usually includes a 2 nucleotide overhang. There is a single processing center in HS Dicer implying that there are two catalytic sites which help form products with the 2 3' overhang. These newly formed segments attach themselves to single stranded mRNA which ultimately leads to mRNA degradation by the cell and translational suppression. The dicer enzyme in humans contains three domains: the <scene name='70/706244/Rnase_iii_1/1'>RNase III 1</scene>, <scene name='70/706244/Rnase_iii_2/1'>RNase III 2</scene>, and the <scene name='70/706244/Paz_domain/1'>Paz Domain</scene>.<ref name=jmol>PMID: 16410517</ref> There are three classes of RNase III proteins which are divided into categories called Escherichia coli RNase III, <scene name='70/706244/Drosha/1'>Drosha</scene>, and Dicer which are given the numbers one, two, and three respectively. The Escherichia coli RNase III class has one domain while the Drosha and dicer have two domains each. There is no evidence of the first class of enzymes in mammals.<ref name=biochem>PMID: 17920623</ref> | ||
| Line 26: | Line 19: | ||
==Pathology== | ==Pathology== | ||
| - | Mutations involving the dicer protein have been linked to the development of diseases in humans. Conditions such as pleuropulmonary blastoma<ref>PMID: 19556464</ref>, goiter multinodular<ref>PMID: 21205968</ref>, and rhabdomyosarcoma<ref>PMID: 21882293</ref> are related to dicer malfunction. Pleuropulmonary blastoma, goiter multinodular, cystic nephroma, and Sertoli-Leydig cell tumors are due a mutation in the Dicer1 gene given the name Dicer1 Syndrome. Dicer1 Syndrome is an inherited disorder that causes the risk of malignant tumors and benign tumors to increase. This occurs because short Dicer proteins are formed that cannot help in the production of miRNA, which can cause cells to grow into tumors. The risk of tumors is mainly increased in the lungs, kidneys, ovaries, and thyroid. Dicer1 Syndrome is transferred in an autosomal dominant pattern. The top treatment is surgery to remove the tumor. | + | Mutations involving the dicer protein have been linked to the development of diseases in humans. Conditions such as pleuropulmonary blastoma<ref>PMID: 19556464</ref>, goiter multinodular<ref>PMID: 21205968</ref>, and rhabdomyosarcoma<ref>PMID: 21882293</ref> are related to dicer malfunction. Pleuropulmonary blastoma, goiter multinodular, cystic nephroma, and Sertoli-Leydig cell tumors are due a mutation in the Dicer1 gene given the name Dicer1 Syndrome. Dicer1 Syndrome is an inherited disorder that causes the risk of malignant tumors and benign tumors to increase. This occurs because short Dicer proteins are formed that cannot help in the production of miRNA, which can cause cells to grow into tumors. The risk of tumors is mainly increased in the lungs, kidneys, ovaries, and thyroid. Dicer1 Syndrome is transferred in an autosomal dominant pattern. The top treatment is surgery to remove the tumor.<ref>PMID: 24761742</ref> |
Dicer is known to be a direct cause of macular degeneration. The absence of Dicer in retinal pigment epithelium causes the eye to break down into macular degeneration. It is hypothesized that Dicer has a specific role in maintaining this retinal health.<ref>PMID: 19836333</ref> | Dicer is known to be a direct cause of macular degeneration. The absence of Dicer in retinal pigment epithelium causes the eye to break down into macular degeneration. It is hypothesized that Dicer has a specific role in maintaining this retinal health.<ref>PMID: 19836333</ref> | ||
| + | |||
| + | For 3D structures of dicer See [[Ribonuclease 3D structures]] | ||
== References == | == References == | ||
<references/> | <references/> | ||
| + | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Justin Woodard, Sam Hayes, Michal Harel, Ann Taylor, Wally Novak, Alexander Berchansky