User:R. Jeremy Johnson/Neurotensin
From Proteopedia
< User:R. Jeremy Johnson(Difference between revisions)
| (3 intermediate revisions not shown.) | |||
| Line 6: | Line 6: | ||
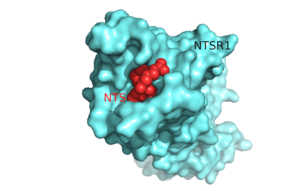
[[Image:Surface_Protein_red.png |300 px|below|thumb|'''Figure 1'''.Top view of NTSR1 protein (blue) interacting with its ligand, NTS(red).]] The neurotensin receptor (NTSR1) belongs to the superfamily of proteins known as [http://proteopedia.org/wiki/index.php/G_protein-coupled_receptor G protein-coupled receptors] (GPCRs). Currently around 800 G protein-coupled receptors have been identified and are hypothesized to be responsible for roughly 80% of [https://en.wikipedia.org/wiki/Signal_transduction signal transduction].<ref name="Millar">PMID:20019124</ref> GPCRs are involved in a vast array of physiological processes within the body that range from interactions with [https://en.wikipedia.org/wiki/Dopamine dopamine] to effects on secretion of bile in the intestines.<ref name="Gui">PMID:11208724</ref> <ref name="Binder">PMID:1173461</ref> Due to the vast array of functions that these proteins serve and their high abundance within the body, these proteins have become major drug targets.<ref name="Fang">PMID:23573662</ref> | [[Image:Surface_Protein_red.png |300 px|below|thumb|'''Figure 1'''.Top view of NTSR1 protein (blue) interacting with its ligand, NTS(red).]] The neurotensin receptor (NTSR1) belongs to the superfamily of proteins known as [http://proteopedia.org/wiki/index.php/G_protein-coupled_receptor G protein-coupled receptors] (GPCRs). Currently around 800 G protein-coupled receptors have been identified and are hypothesized to be responsible for roughly 80% of [https://en.wikipedia.org/wiki/Signal_transduction signal transduction].<ref name="Millar">PMID:20019124</ref> GPCRs are involved in a vast array of physiological processes within the body that range from interactions with [https://en.wikipedia.org/wiki/Dopamine dopamine] to effects on secretion of bile in the intestines.<ref name="Gui">PMID:11208724</ref> <ref name="Binder">PMID:1173461</ref> Due to the vast array of functions that these proteins serve and their high abundance within the body, these proteins have become major drug targets.<ref name="Fang">PMID:23573662</ref> | ||
| - | The ligand for NTSR1 is the 13 amino acid peptide, neurotensin (NTS)<ref name="SONT">PMID:23051748</ref>, and the majority of the effects of NTS are mediated through NTSR1<ref name="SONT"/>. NTS has a variety of biological activities including a role in the '''[https://en.wikipedia.org/wiki/Leptin leptin]''' signaling pathways <ref name="Mice">PMID: 20211191</ref>, tumor growth <ref name="cancer">PMID:16887236</ref>, and '''[https://en.wikipedia.org/wiki/Dopamine dopamine]''' regulation <ref name="Schizophrenia">PMID:22596253</ref>. NTSR1 was crystallized bound with a C-terminal portion of its tridecapeptide '''[https://en.wikipedia.org/wiki/Ligand ligand]''', <scene name='72/721548/Neurotensin/7'>NTS(8-13)</scene>. The shortened ligand was used because | + | The ligand for NTSR1 is the 13 amino acid peptide, neurotensin (NTS)<ref name="SONT">PMID:23051748</ref>, and the majority of the effects of NTS are mediated through NTSR1<ref name="SONT"/>. NTS has a variety of biological activities including a role in the '''[https://en.wikipedia.org/wiki/Leptin leptin]''' signaling pathways <ref name="Mice">PMID: 20211191</ref>, tumor growth <ref name="cancer">PMID:16887236</ref>, and '''[https://en.wikipedia.org/wiki/Dopamine dopamine]''' regulation <ref name="Schizophrenia">PMID:22596253</ref>. NTSR1 was crystallized bound with a C-terminal portion of its tridecapeptide '''[https://en.wikipedia.org/wiki/Ligand ligand]''', <scene name='72/721548/Neurotensin/7'>NTS(8-13)</scene>. The shortened ligand was used because of oits higher potency and efficacy than its full-length counterpart<ref name="SONT"/>. |
| - | A critical topic in the understanding of GPCRs is the transition from the inactive to active state. This transition is responsible for the [https://en.wikipedia.org/wiki/Signal_transduction transduction] of a signal from the extracellular to the intracellular space. The transition occurs when a ligand, NTS in the case of NTSR1, binds to the receptor causing a [https://en.wikipedia.org/wiki/Conformational_change conformational change] | + | A critical topic in the understanding of GPCRs is the transition from the inactive to active state. This transition is responsible for the [https://en.wikipedia.org/wiki/Signal_transduction transduction] of a signal from the extracellular to the intracellular space. The transition occurs when a ligand, NTS in the case of NTSR1, binds to the receptor causing a [https://en.wikipedia.org/wiki/Conformational_change conformational change] that leads to the activation of the intracellular G protein. Currently, only the structure of the active form of NTSR1 is known, making the transition between the active and inactive states difficult to study.<ref name="SONT"/> |
== Neurotensin == | == Neurotensin == | ||
| Line 14: | Line 14: | ||
== Structure == | == Structure == | ||
| - | Like other G protein-coupled receptors, | + | Like other G protein-coupled receptors, NTSR1 is composed of 3 distinct regions. An <scene name='72/727765/Overall_structure/5'>extracellular binding site</scene> where neurotensin binds and causes a conformational change of the protein. A region containing <scene name='72/727765/Overall_structure/4'>7 transmembrane alpha helices</scene> (PDB code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4GRV 4GRV)] that transduce the signal from the extracellular side of the cell membrane to the intracellular side. Lastly, an intracellular region that when activated by a conformational change in the protein activates a [https://en.wikipedia.org/wiki/G_protein G-protein] associated with this receptor. |
| - | The <scene name='72/721547/Hydrophobic_binding_pocket/6'>hydrophobic binding pocket</scene> in NTSR1 is located at the top of the protein (Figure 1). NTSR1 also contains an '''[https://en.wikipedia.org/wiki/Allosteric_regulation allosteric]''' <scene name='72/721548/Na_bind_pocket/13'>sodium binding pocket</scene>, which is located directly beneath the ligand binding pocket and the two pockets are separated by the residue <scene name='72/721548/Trp321/1'>Trp321</scene><ref name="SPGP">PMID:26205105</ref>. NTSR1 has been mutated to exist in both <scene name='72/721548/Ntsr1-elf/6'>active</scene> and <scene name='72/721547/Ntsr1-gw5/8'>active-like</scene> states. | + | The <scene name='72/721547/Hydrophobic_binding_pocket/6'>hydrophobic binding pocket</scene> in NTSR1 is located at the top of the protein (Figure 1). NTSR1 also contains an '''[https://en.wikipedia.org/wiki/Allosteric_regulation allosteric]''' <scene name='72/721548/Na_bind_pocket/13'>sodium binding pocket</scene>, which is located directly beneath the ligand binding pocket and the two pockets, which are separated by the residue <scene name='72/721548/Trp321/1'>Trp321</scene><ref name="SPGP">PMID:26205105</ref>. NTSR1 has been mutated to exist in both <scene name='72/721548/Ntsr1-elf/6'>active</scene> and <scene name='72/721547/Ntsr1-gw5/8'>active-like</scene> states. |
===Neurotensin Binding Pocket=== | ===Neurotensin Binding Pocket=== | ||
On the extracellular side of the protein is the | On the extracellular side of the protein is the | ||
| - | <scene name='72/721547/Hydrophobic_binding_pocket/6'>hydrophobic binding pocket</scene>. <ref name="SONT"/>Binding of NTS to NTSR1 is enriched by <scene name='72/721539/Binding_pocket_surface/4'>charge complementarity</scene> between the <font color='#0000CD'>positive</font> NTS arginine side chains and the [https://en.wikipedia.org/wiki/Electronegativity <font color='#FF0000'>negative</font>] pocket. In addition, the C-terminus of <font color='#32CD32'>NTS</font> forms a <scene name='72/721539/Binding_site_charges/4'>salt bridge</scene> (PDB code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4GRV 4GRV)] with Arg328 of <font color='#A9A9A9'>NTSR1</font>. Three [https://en.wikipedia.org/wiki/Hydrogen_bond hydrogen bonds] are made between the side chains of NTS and the receptor while most of the interactions are a result of [https://en.wikipedia.org/wiki/Van_der_Waals_force Van der Waals] interactions. The binding pocket is partially capped by a <scene name='72/721539/B-hairpin_loop/1'>Β-hairpin loop</scene> at the proximal end of the receptor's N-terminus.<ref name=" | + | <scene name='72/721547/Hydrophobic_binding_pocket/6'>hydrophobic binding pocket</scene>. <ref name="SONT"/>Binding of NTS to NTSR1 is enriched by <scene name='72/721539/Binding_pocket_surface/4'>charge complementarity</scene> between the <font color='#0000CD'>positive</font> NTS arginine side chains and the [https://en.wikipedia.org/wiki/Electronegativity <font color='#FF0000'>negative</font>] pocket. In addition, the C-terminus of <font color='#32CD32'>NTS</font> forms a <scene name='72/721539/Binding_site_charges/4'>salt bridge</scene> (PDB code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4GRV 4GRV)] with Arg328 of <font color='#A9A9A9'>NTSR1</font>. Three [https://en.wikipedia.org/wiki/Hydrogen_bond hydrogen bonds] are made between the side chains of NTS and the receptor while most of the interactions are a result of [https://en.wikipedia.org/wiki/Van_der_Waals_force Van der Waals] interactions. The binding pocket is partially capped by a <scene name='72/721539/B-hairpin_loop/1'>Β-hairpin loop</scene> at the proximal end of the receptor's N-terminus.<ref name="SONT"/> |
| - | The binding of NTS to NTSR1 is also driven by hydrophobic stacking. One key residue in this pocket is a Phenylalanine at position 358, which takes part in a network of hydrophobic stacking interactions<ref name="SPGP"/>. These interactions stabilize the Trp321 and Tyr324 residues allowing Tyr324 to interact with the [https://en.wikipedia.org/wiki/C-terminus C-terminal] <scene name='72/721547/Ligand_protein_interactions/8'>Leu13 residue of NTS ligand</scene> via [https://en.wikipedia.org/wiki/Van_der_Waals_force Van der Waals interactions].<ref name="SONT"/><ref name="SPGP"/> Without the hydrophobic stacking interactions that are facilitated by the Phe358, this binding interaction would be destabilized. Trp321 also participates in these stacking interactions and serves as the boundary between the ligand binding pocket and the Na<sup>+</sup> binding pocket.<ref name="SPGP"/> Another major player in the transduction of the extracellular signal to the intracellular G-protein is the <scene name='72/727765/Hydrogen_bonding_network/4'>hydrogen bonding network</scene> (PDB code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4GRV 4GRV)]that links the bound <font color='#32CD32'>hormone</font> with the [https://en.wikipedia.org/wiki/Hydrophobe hydrophobic] core of the <font color='#A9A9A9'>neurotensin receptor</font>. The carboxylate of Leu13 of NTS forms a hydrogen bond network with Arg327, Arg328, and Tyr324. The Tyr324, in turn, is brought into an orientation to make the formation of a <scene name='72/727765/Hydrophobic_stacking_4xee/2'>hydrophobic stacking</scene> (PDB Code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4XEE 4XEE]) network between Phe358, Trp321, Ala157, and Phe317 possible.<ref name=" | + | The binding of NTS to NTSR1 is also driven by hydrophobic stacking. One key residue in this pocket is a Phenylalanine at position 358, which takes part in a network of hydrophobic stacking interactions<ref name="SPGP"/>. These interactions stabilize the Trp321 and Tyr324 residues allowing Tyr324 to interact with the [https://en.wikipedia.org/wiki/C-terminus C-terminal] <scene name='72/721547/Ligand_protein_interactions/8'>Leu13 residue of NTS ligand</scene> via [https://en.wikipedia.org/wiki/Van_der_Waals_force Van der Waals interactions].<ref name="SONT"/><ref name="SPGP"/> Without the hydrophobic stacking interactions that are facilitated by the Phe358, this binding interaction would be destabilized. Trp321 also participates in these stacking interactions and serves as the boundary between the ligand binding pocket and the Na<sup>+</sup> binding pocket.<ref name="SPGP"/> Another major player in the transduction of the extracellular signal to the intracellular G-protein is the <scene name='72/727765/Hydrogen_bonding_network/4'>hydrogen bonding network</scene> (PDB code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4GRV 4GRV)]that links the bound <font color='#32CD32'>hormone</font> with the [https://en.wikipedia.org/wiki/Hydrophobe hydrophobic] core of the <font color='#A9A9A9'>neurotensin receptor</font>. The carboxylate of Leu13 of NTS forms a hydrogen bond network with Arg327, Arg328, and Tyr324. The Tyr324, in turn, is brought into an orientation to make the formation of a <scene name='72/727765/Hydrophobic_stacking_4xee/2'>hydrophobic stacking</scene> (PDB Code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4XEE 4XEE]) network between Phe358, Trp321, Ala157, and Phe317 possible.<ref name="SONT"/> The effects that this network has on the activation of the intracellular G-protein was examined by the [https://en.wikipedia.org/wiki/Mutagenesis mutagenesis] of amino acids that disrupted the formation of this network. Mutagenesis of <scene name='72/727765/Overall_structure/12'>A86L</scene>, <scene name='72/727765/Overall_structure/13'>E166A</scene>, <scene name='72/727765/Overall_structure/14'>G125L</scene>, <scene name='72/727765/Overall_structure/15'>L310A</scene>, <scene name='72/727765/Overall_structure/16'>F358A</scene>, and <scene name='72/727765/Overall_structure/17'>V360A</scene> showed that when this interaction was disrupted, the receptor no longer activated the intracellular G-protein.<ref name="SONT"/> This discovery lead to the conclusion that the conformational changes caused by this stacking allows for the signal to be moved from the extracellular binding site through the transmembrane helices of the receptor to the intracellular region activating the G-protein. |
===Na<sup>+</sup> Binding Pocket=== | ===Na<sup>+</sup> Binding Pocket=== | ||
[[Image:4XEE closed sodium pocket.png|250 px|right|thumb|Figure 2: Closed form of sodium binding pocket that caps the entrance of sodium into the top of the binding pocket. (PDB Code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4XEE 4XEE])]] | [[Image:4XEE closed sodium pocket.png|250 px|right|thumb|Figure 2: Closed form of sodium binding pocket that caps the entrance of sodium into the top of the binding pocket. (PDB Code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4XEE 4XEE])]] | ||
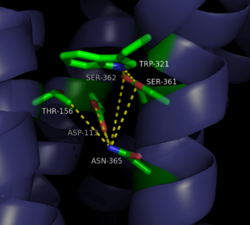
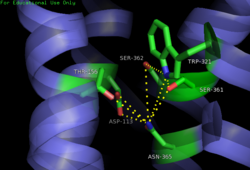
| - | [[Image:4GRV open binding pocket.png|250 px|right|thumb|Figure 3: Open form of sodium binding pocket that does not cap the entrance of sodium into the top of the binding pocket. (PDB code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4GRV 4GRV])]] Conserved across all class A GPCRs, a <scene name='72/727765/Sodium_binding_pocket_final/1'>sodium binding pocket</scene> (PDB code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4GRV 4GRV]) is seen in the middle of TM2 helix. The bound sodium ion is coordinated with a highly conserved Asp113 and four other oxygen contacts from a combination of water molecules. For G-protein activation, a hydrogen bond coordination with | + | [[Image:4GRV open binding pocket.png|250 px|right|thumb|Figure 3: Open form of sodium binding pocket that does not cap the entrance of sodium into the top of the binding pocket. (PDB code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4GRV 4GRV])]] Conserved across all class A GPCRs, a <scene name='72/727765/Sodium_binding_pocket_final/1'>sodium binding pocket</scene> (PDB code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4GRV 4GRV]) is seen in the middle of TM2 helix of NTSR1. The bound sodium ion is coordinated with a highly conserved Asp113 and four other oxygen contacts from a combination of water molecules. For G-protein activation, a hydrogen bond coordination with Thr156, Ser362, and Asn365 of the NPxxY [https://en.wikipedia.org/wiki/Structural_motif motif] must occur. Trp321 helps to maintain the active conformation of the receptor by occluding the top of the binding pocket using [https://en.wikipedia.org/wiki/Van_der_Waals_force Van der Waals] interactions (Figure 2). This occlusion stops sodium ions from entering the top of the binding pocket and helps NTSR1 remain in its active conformation. The conformation of the binding pocket where Trp321 does not occlude the top can be seen when mutations to A86L, G215A, and V360A are present (Figure 3). This form of the receptor allows more sodium into the binding pocket and stabilizes the inactive form of the receptor.<ref name="Katritch">PMID:24767681</ref> <scene name='72/721548/Trp321/1'>Trp321</scene>, which is positioned at the bottom of the <scene name='72/721547/Hydrophobic_binding_pocket/5'>hydrophobic binding pocket</scene>, sets the top of the <scene name='72/721548/Na_bind_pocket/12'> sodium binding pocket</scene>. The Na<sup>+</sup> ion binding pocket acts as a negative allosteric site for G protein activity.<ref name="SPGP"/> When Na<sup>+</sup> enters the Na<sup>+</sup> ion binding pocket, it coordinates with Asp95, Gln131, Ser135, and Asp113, decreasing the signaling activity of NTSR1 <ref name="SPGP"/>. When NTSR1 is in its active state, the Na<sup>+</sup> ion binding pocket is collapsed. This prevents the regulation of protein activity through a Na<sup>+</sup> ion, as the Na<sup>+</sup> ion is unable to coordinate via a salt bridge to Asp113 (Figure 3). The side chain atoms of Asp113 form a '''[https://en.wikipedia.org/wiki/Hydrogen_bond hydrogen bond]''' network with Thr156, Ser361, Ser362, and Gln365 instead, which prevents the coordination of a Na<sup>+</sup> ion<ref name ="SPGP"/> (Figure 3). |
==Activation of NTSR1== | ==Activation of NTSR1== | ||
| - | Since wild type NTSR1 was unstable in detergent solution | + | Since wild type NTSR1 was unstable in detergent solution, six residues in the protein were mutated for stabilization.<ref name="SONT"/> <ref name="SPGP"/> |
===Active-Like State=== | ===Active-Like State=== | ||
| - | The six amino acid mutations for thermostabilization <ref name="SONT"/> were <scene name='72/727765/Overall_structure/12'>A86L</scene>, <scene name='72/727765/Overall_structure/13'>E166A</scene>, <scene name='72/727765/Overall_structure/14'>G125L</scene>, <scene name='72/727765/Overall_structure/15'>L310A</scene>, <scene name='72/727765/Overall_structure/16'>F358A</scene>, and <scene name='72/727765/Overall_structure/17'>V360A</scene>. This protein was found to have NTS affinity similar to that of wild tpye NTSR1, and was named <scene name='72/721547/Ntsr1-gw5/8'>NTSR1-GW5</scene>. | + | The six amino acid mutations for thermostabilization <ref name="SONT"/> were <scene name='72/727765/Overall_structure/12'>A86L</scene>, <scene name='72/727765/Overall_structure/13'>E166A</scene>, <scene name='72/727765/Overall_structure/14'>G125L</scene>, <scene name='72/727765/Overall_structure/15'>L310A</scene>, <scene name='72/727765/Overall_structure/16'>F358A</scene>, and <scene name='72/727765/Overall_structure/17'>V360A</scene>. This protein was found to have NTS affinity similar to that of wild tpye NTSR1, and was named <scene name='72/721547/Ntsr1-gw5/8'>NTSR1-GW5</scene>. However, NTSR1-GW5 did not have G-protein activity.<ref name="SONT"/> |
===Active State=== | ===Active State=== | ||
| - | After determining | + | After determining that <scene name='72/721547/Ntsr1-gw5/8'>NTSR1-GW5</scene> was only active-like, NTSR1 was recrystallized in a more fully active state.<ref name="SPGP"/> By reverting back three of the original six mutations, NTSR1 regained near wild-type G-protein activity.<ref name="SPGP"/> The three reversions were Asp166, Leu310, and Phe358, and this protein was named <scene name='72/721548/Ntsr1-elf/6'>NTSR1-ELF</scene>. The revival of activity in NTSR1 indicated that the reverted amino acid residues (Asp166, Leu310, and Phe358) play significant roles in G-protein activity.<ref name="SPGP"/> |
====Leu310==== | ====Leu310==== | ||
| Line 52: | Line 52: | ||
===Cancer Studies=== | ===Cancer Studies=== | ||
| - | Some tumor cells can secrete and express NTS and NTS receptors | + | Some tumor cells can secrete and express NTS and NTS receptors, suggesting that NTS '''[https://en.wikipedia.org/wiki/Autocrine_signalling autocrine]''', '''[https://en.wikipedia.org/wiki/Endocrine_system endocrine]''' and '''[https://en.wikipedia.org/wiki/Paracrine_signalling paracrine]''' regulation are possible. This leads to aggressive growth and tumor development. Injecting animals with NTS increased tumor growth and size, while injecting them with NTS antagonist had the opposite effect.<ref name="cancer"/> |
===Dopamine Regulation=== | ===Dopamine Regulation=== | ||
| Line 60: | Line 60: | ||
[[Image: Meclinerant.jpg |200 px|right|thumb|Figure 4: Meclinerant: An inhibitor of NTSR1 found to enhance selectivity of radiotherapy in cancer treatment.]] NTSR1 is commonly expressed in various invasive [https://en.wikipedia.org/wiki/Cancer cancer] cell lines making it a promising cancer drug target. It is prevalent in [https://en.wikipedia.org/wiki/Colorectal_cancer colon cancer] [https://en.wikipedia.org/wiki/Adenocarcinoma adenocarcinoma], but is not found in adult colon cell types.<ref name="Valerie">PMID:21903767</ref> NTSR1 is also found in aggressive [https://en.wikipedia.org/wiki/Prostate_cancer prostate cancer] cells, but not [https://en.wikipedia.org/wiki/Epithelium epithelial] prostate cells. In prostate cancer cells, binding of NTS results in [https://en.wikipedia.org/wiki/Mitogen-activated_protein_kinase mitogen-activated protein kinase (PKB)], [https://en.wikipedia.org/wiki/Phosphoinositide_3-kinase phosphoinositide-3 kinase (PI-3K)], [https://en.wikipedia.org/wiki/Epidermal_growth_factor_receptor epidermal growth factor receptor (EGFR)], [https://en.wikipedia.org/wiki/Proto-oncogene_tyrosine-protein_kinase_Src SRC], and [https://en.wikipedia.org/wiki/STAT5 STAT5] phosphorylation.<ref name="Valerie"/> These all result in increased DNA synthesis, [https://en.wikipedia.org/wiki/Cell_growth cell proliferation], and survival. Inhibition of NTSR1 and its downstream signaling represents a target for [https://en.wikipedia.org/wiki/Radiation_therapy radiotherapy], which uses radiation to target malignant cells. NTSR1 can be inhibited by agonist [https://en.wikipedia.org/wiki/Meclinertant meclinertant] which inhibits proliferation and prosurvival of cancer cells. Combination treatment of radiation and meclinerant provides selective treatment of cancer cells over normal cells, indicating the need for clinical trials of this approach. <ref name="Kisfalvi">PMID:19679549</ref> | [[Image: Meclinerant.jpg |200 px|right|thumb|Figure 4: Meclinerant: An inhibitor of NTSR1 found to enhance selectivity of radiotherapy in cancer treatment.]] NTSR1 is commonly expressed in various invasive [https://en.wikipedia.org/wiki/Cancer cancer] cell lines making it a promising cancer drug target. It is prevalent in [https://en.wikipedia.org/wiki/Colorectal_cancer colon cancer] [https://en.wikipedia.org/wiki/Adenocarcinoma adenocarcinoma], but is not found in adult colon cell types.<ref name="Valerie">PMID:21903767</ref> NTSR1 is also found in aggressive [https://en.wikipedia.org/wiki/Prostate_cancer prostate cancer] cells, but not [https://en.wikipedia.org/wiki/Epithelium epithelial] prostate cells. In prostate cancer cells, binding of NTS results in [https://en.wikipedia.org/wiki/Mitogen-activated_protein_kinase mitogen-activated protein kinase (PKB)], [https://en.wikipedia.org/wiki/Phosphoinositide_3-kinase phosphoinositide-3 kinase (PI-3K)], [https://en.wikipedia.org/wiki/Epidermal_growth_factor_receptor epidermal growth factor receptor (EGFR)], [https://en.wikipedia.org/wiki/Proto-oncogene_tyrosine-protein_kinase_Src SRC], and [https://en.wikipedia.org/wiki/STAT5 STAT5] phosphorylation.<ref name="Valerie"/> These all result in increased DNA synthesis, [https://en.wikipedia.org/wiki/Cell_growth cell proliferation], and survival. Inhibition of NTSR1 and its downstream signaling represents a target for [https://en.wikipedia.org/wiki/Radiation_therapy radiotherapy], which uses radiation to target malignant cells. NTSR1 can be inhibited by agonist [https://en.wikipedia.org/wiki/Meclinertant meclinertant] which inhibits proliferation and prosurvival of cancer cells. Combination treatment of radiation and meclinerant provides selective treatment of cancer cells over normal cells, indicating the need for clinical trials of this approach. <ref name="Kisfalvi">PMID:19679549</ref> | ||
| - | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
| + | |||
| + | ==Proteopedia Resources== | ||
| + | [http://proteopedia.org/wiki/index.php/Category:Neurotensin Category:Neurotensin] | ||
| + | |||
| + | [http://proteopedia.org/wiki/index.php/Category:Neurotensin_receptor Category:Neurotensin receptor] | ||
| + | |||
| + | [http://proteopedia.org/wiki/index.php/User:R._Jeremy_Johnson/CH462:Biochemistry_II_Butler_University Butler University Structures] | ||
| + | |||
| + | </StructureSection> | ||
| + | |||
| + | |||
| + | ==Student Contributors== | ||
| + | Allie Cotter | ||
| + | Danny Cotter | ||
| + | Andrew Koelper | ||
| + | Brent Waibel | ||
Current revision
Student Contributors
Allie Cotter Danny Cotter Andrew Koelper Brent Waibel