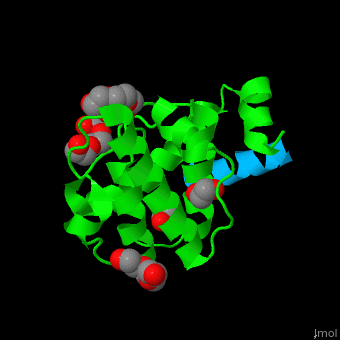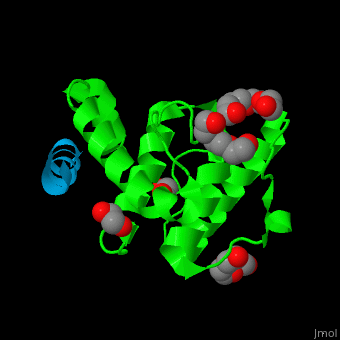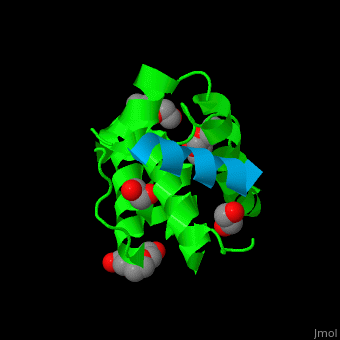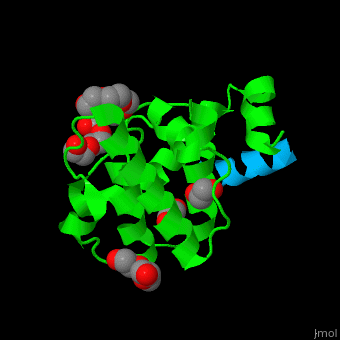
Paxillin
From Proteopedia
(Difference between revisions)
| (6 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | <StructureSection load='2vzi' size=' | + | <StructureSection load='2vzi' size='400' side='right' caption='Human paxillin LD4 domain complex (blue) with α-parvin (green), tetraethylene glycol, triethylene glycol and etylene glycol (PDB code [[2vzi]])' scene='71/715908/Cv/1' pspeed='8'> |
== Function == | == Function == | ||
'''Paxillin''' (PXN) is involved in actin membrane attachment at sites of cell adhesion to focal adhesion domains. PXN contains a number of domains which are involved in protein-protein interactions: LD, LIM, SH2 and SH3 binding sites. LD motifs are leucine-rich sequences which begin with leucine (L) and end with aspartate (D)<ref>PMID:11911889</ref>. PXN serves as a docking protein recruiting signaling molecules to focal adhesions. | '''Paxillin''' (PXN) is involved in actin membrane attachment at sites of cell adhesion to focal adhesion domains. PXN contains a number of domains which are involved in protein-protein interactions: LD, LIM, SH2 and SH3 binding sites. LD motifs are leucine-rich sequences which begin with leucine (L) and end with aspartate (D)<ref>PMID:11911889</ref>. PXN serves as a docking protein recruiting signaling molecules to focal adhesions. | ||
== Disease == | == Disease == | ||
| - | PXN has enhanced expression in several types of cancer PXN mutations are associated with lung cancer tumor growth<ref>PMID:18172305</ref>. | + | PXN has enhanced expression in several types of cancer. PXN mutations are associated with lung cancer tumor growth<ref>PMID:18172305</ref>. |
== Structural highlights == | == Structural highlights == | ||
PXN LD motifs are localized on the N-terminal region while the LIM double zinc finger domains are found at the C-terminal. | PXN LD motifs are localized on the N-terminal region while the LIM double zinc finger domains are found at the C-terminal. | ||
| - | + | *<scene name='71/715908/Cv/2'>Human paxillin LD4 domain interactions with α-parvin</scene>. | |
</StructureSection> | </StructureSection> | ||
| Line 16: | Line 16: | ||
{{#tree:id=OrganizedByTopic|openlevels=0| | {{#tree:id=OrganizedByTopic|openlevels=0| | ||
| - | *Paxillin LD1 | + | *Paxillin LD1 (residues 1-20) |
**[[4edn]] – hPXN + beta-parvin CH2 domain - human<br /> | **[[4edn]] – hPXN + beta-parvin CH2 domain - human<br /> | ||
**[[2k2r]] – hPXN + beta-parvin CH2 domain - NMR<br /> | **[[2k2r]] – hPXN + beta-parvin CH2 domain - NMR<br /> | ||
**[[2vzg]], [[2vzd]] – hPXN + alpha-parvin C terminal <br /> | **[[2vzg]], [[2vzd]] – hPXN + alpha-parvin C terminal <br /> | ||
| - | **[[3rqe]] – hPXN + CCM3<br /> | + | **[[3rqe]], [[3rqg]], [[3rqf]] – hPXN + CCM3<br /> |
| + | **[[1ow6]], [[3gm1]] – hPXN + tyrosine kinase PYK2 focal adhesion targeting domain <br /> | ||
| + | **[[4xgz]], [[4xh2]] – hPXN + antibody<br /> | ||
| + | **[[1ow8]], [[1ow7]] – hPXN + focal adhesion kinase focal adhesion targeting domain <br /> | ||
| + | **[[5w93]] – mPXN + P130CAS - mouse<br /> | ||
| - | *Paxillin | + | *Paxillin LD4 (residues 20-41) |
| - | **[[ | + | **[[6iui]] – hPXN + GIT1 PBD domain<br /> |
| - | + | ||
| - | + | ||
| - | + | ||
| - | *Paxillin | + | *Paxillin (residues 139-162) |
**[[4r32]] – cPXN + tyrosine kinase PYK2 focal adhesion targeting domain - chicken<br /> | **[[4r32]] – cPXN + tyrosine kinase PYK2 focal adhesion targeting domain - chicken<br /> | ||
| - | **[[1ow6]], [[3gm1]] – hPXN + tyrosine kinase PYK2 focal adhesion targeting domain <br /> | ||
| - | **[[1ow7]] – hPXN + focal adhesion kinase focal adhesion targeting domain <br /> | ||
**[[2l6f]] – hPXN + focal adhesion kinase focal adhesion targeting domain - NMR<br /> | **[[2l6f]] – hPXN + focal adhesion kinase focal adhesion targeting domain - NMR<br /> | ||
| - | **[[4xh2]] – hPXN + antibody<br /> | ||
**[[2vzi]] – hPXN + alpha-parvin C terminal <br /> | **[[2vzi]] – hPXN + alpha-parvin C terminal <br /> | ||
| - | **[[3rqg]] – hPXN + CCM3<br /> | ||
| - | *Paxillin proline-rich region | + | *Paxillin (residues 260-281) |
| + | |||
| + | **[[5uwh]] – hPXN + RAN + CRM1 + RANBP1<br /> | ||
| + | **[[3u3f]] – hPXN + tyrosine kinase PYK2 focal adhesion domain<br /> | ||
| + | **[[6jmu]] – mPXN + GIT1<br /> | ||
| + | |||
| + | *Paxillin proline-rich region (residues 45-54) | ||
**[[2o9v]] – hPXN + ponsin SH3 domain<br /> | **[[2o9v]] – hPXN + ponsin SH3 domain<br /> | ||
| + | |||
| + | *Paxillin LIM4 domain (residues 527-591) | ||
| + | |||
| + | **[[6u4m]] – hPXN - NMR<br /> | ||
| + | **[[6u4n]] – hPXN + kindling-2 - NMR<br /> | ||
| + | |||
}} | }} | ||
== References == | == References == | ||
<references/> | <references/> | ||
| + | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
3D structures of paxillin
Updated on 17-September-2020
References
- ↑ Tumbarello DA, Brown MC, Turner CE. The paxillin LD motifs. FEBS Lett. 2002 Feb 20;513(1):114-8. PMID:11911889
- ↑ Jagadeeswaran R, Surawska H, Krishnaswamy S, Janamanchi V, Mackinnon AC, Seiwert TY, Loganathan S, Kanteti R, Reichman T, Nallasura V, Schwartz S, Faoro L, Wang YC, Girard L, Tretiakova MS, Ahmed S, Zumba O, Soulii L, Bindokas VP, Szeto LL, Gordon GJ, Bueno R, Sugarbaker D, Lingen MW, Sattler M, Krausz T, Vigneswaran W, Natarajan V, Minna J, Vokes EE, Ferguson MK, Husain AN, Salgia R. Paxillin is a target for somatic mutations in lung cancer: implications for cell growth and invasion. Cancer Res. 2008 Jan 1;68(1):132-42. doi: 10.1158/0008-5472.CAN-07-1998. PMID:18172305 doi:http://dx.doi.org/10.1158/0008-5472.CAN-07-1998