We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Green Fluorescent Protein
From Proteopedia
(Difference between revisions)
| (38 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
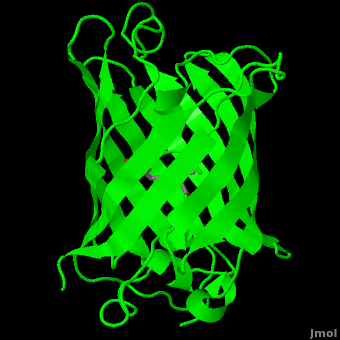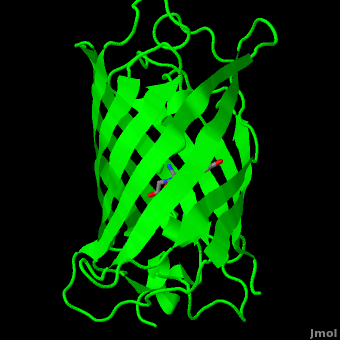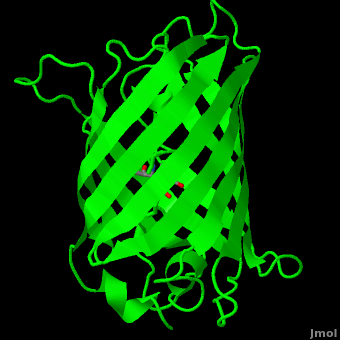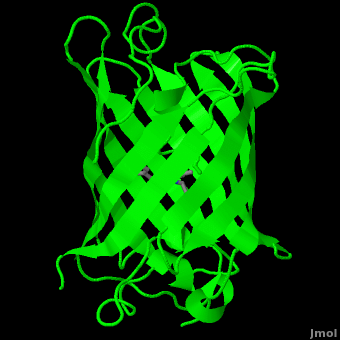
<StructureSection load='1ema' size='350' side='right' scene='Green_Fluorescent_Protein/1ema_gfp_default/2' caption='Green fluorescent protein complex with peptide-derived chromophore ([[1ema]])' > | <StructureSection load='1ema' size='350' side='right' scene='Green_Fluorescent_Protein/1ema_gfp_default/2' caption='Green fluorescent protein complex with peptide-derived chromophore ([[1ema]])' > | ||
==Function== | ==Function== | ||
| - | '''Green fluorescent protein (GFP)''' is a | + | '''Green fluorescent protein (GFP)''' is a bioluminescent polypeptide consisting of 238 residues isolated from the body of ''Aequorea victoria'' jellyfish.<ref name="PDBsum">[http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=1ema&template=main.html], Protein Database (PDBsum): 1ema. European Bioinformatics (EBI); 2009.</ref> GFP converts the blue chemiluminescent of aequorin in the jellyfish into green fluorescent light.<ref name="Yang">[http://www-bioc.rice.edu/Bioch/Phillips/Papers/gfpbio.html], Yang F, Moss LG, Phillips GN Jr. 1996. The molecular structure of green fluorescent protein. Biotechnology. 14: 1246-1251. DOI 10.1038/nbt1096-1246.</ref> It remains unclear why these jellyfish use fluorescence, why green is better than blue, or why they produce a separate protein for green fluorescence as opposed to simply mutating the present aequorin to shift its wavelength,<ref name="Tsien" /> but in the laboratory, GFP can be incorporated into a variety of biological systems in order to function as a marker protein. Since its discovery in 1962, GFP has come to play a significant role in research as a tool to monitor gene expression, cellular localization, protein mobility, intracellular trafficking, and interactions between various membrane and cytoplasmic proteins, as well as many others. |
| + | * '''Superfolder GFP''' does not misfold when fused to other proteins. | ||
| + | *'''Photoconvertible fluorescent protein''' changes the emission when exposed to UV light<ref>PMID:32242924</ref>. | ||
| + | |||
| + | See also<br /> | ||
[[Green Fluorescent Protein: Research Tool]].<ref name="Haldar"> [http://www.springerlink.com/content/wvg513864266g77n/fulltext.pdf], Haldar S, Chattopadhyay A. 2009. The green journey. J Fluoresc. 19:1-2. DOI 10.1007/s10895-008-0455-6; biographical background on [http://en.wikipedia.org/wiki/Douglas_Prasher Douglas Prasher], [http://en.wikipedia.org/wiki/Martin_Chalfie Martin Chalfie] and [http://en.wikipedia.org/wiki/Roger_Tsien Roger Tsien].</ref><br /> | [[Green Fluorescent Protein: Research Tool]].<ref name="Haldar"> [http://www.springerlink.com/content/wvg513864266g77n/fulltext.pdf], Haldar S, Chattopadhyay A. 2009. The green journey. J Fluoresc. 19:1-2. DOI 10.1007/s10895-008-0455-6; biographical background on [http://en.wikipedia.org/wiki/Douglas_Prasher Douglas Prasher], [http://en.wikipedia.org/wiki/Martin_Chalfie Martin Chalfie] and [http://en.wikipedia.org/wiki/Roger_Tsien Roger Tsien].</ref><br /> | ||
| - | [[Colored & Bioluminescent Protein]]<br />In Hebrew: [[GFP (Hebrew)]] and [[Gfp vc2]]. | + | [[Colored & Bioluminescent Protein]]<br /> |
| + | For''' red fluorescent protein''' see [[MCherry Fluorescent Protein]].<br /> | ||
| + | In Hebrew: [[GFP (Hebrew)]] and [[Gfp vc2]]. | ||
==History== | ==History== | ||
| Line 11: | Line 17: | ||
Shimomura was originally looking only to isolate the blue luminescent protein of ''Aequorea victoria'', traditionally thought to be [[luciferase]], but it would soon become apparent that the glow was in fact due to aequorin, a substance related, but slightly varying from luciferase.<ref name="Haldar" /><ref name="Shimomura" /> However, the light emitted from aequorin still differed from the light emitted from the wild jellyfish. This quandary led to the discovery of the green fluorescent protein responsible for this disparity, but sufficient amounts of the protein could not be collected for study until 1979. The journey to discover the nature of GFP had begun.<ref name="Shimomura" /> | Shimomura was originally looking only to isolate the blue luminescent protein of ''Aequorea victoria'', traditionally thought to be [[luciferase]], but it would soon become apparent that the glow was in fact due to aequorin, a substance related, but slightly varying from luciferase.<ref name="Haldar" /><ref name="Shimomura" /> However, the light emitted from aequorin still differed from the light emitted from the wild jellyfish. This quandary led to the discovery of the green fluorescent protein responsible for this disparity, but sufficient amounts of the protein could not be collected for study until 1979. The journey to discover the nature of GFP had begun.<ref name="Shimomura" /> | ||
| - | In the 1990’s, Douglas Prasher, Frank | + | In the 1990’s, Douglas Prasher, Frank Prendergast, and co-workers successfully cloned the gene that encoded for GFP. Martin Chalfie further pursued this line of work and was eventually able to express GFP in heterologous systems such as E. coli and C. elegans. Chalfie’s research provided the first evidence that GFP was unique as it did not require the presence of any exogenous substance or cofactor for fluorescence.<ref name="Haldar" /> The lack for the need for a cofactor proved that the cloned GFP gene contained all the information necessary for posttranslational synthesis of the chromophore. <ref name="Tsien" /> |
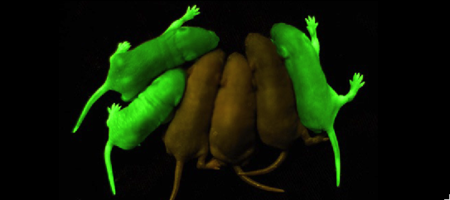
Roger Tsien and co-workers were intrigued by the absence of a necessary cofactor and began to research the structure of GFP and how it relates to its fluorescence. They discovered that a helix within the beta barrel structure of GFP actually contained a fluorophore responsible for fluorescence. In researching its structure, they were able to develop GFP derivatives with improved fluorescence and photo-stability. Shimomura, Chalfie, and Tsien were each recognized for their work involving GFP with the Nobel Prize in 2008.<ref name="Haldar" /> In the time since the work of these three researchers, GFP has been successfully expressed and utilized in bacteria, yeast, slime mold, plants, drosophila fruit flies, zebra-fish, and mammalian cells.<ref name="Yang" /> Below, mice have had GFP inserted into their genomes for studies in neurology. | Roger Tsien and co-workers were intrigued by the absence of a necessary cofactor and began to research the structure of GFP and how it relates to its fluorescence. They discovered that a helix within the beta barrel structure of GFP actually contained a fluorophore responsible for fluorescence. In researching its structure, they were able to develop GFP derivatives with improved fluorescence and photo-stability. Shimomura, Chalfie, and Tsien were each recognized for their work involving GFP with the Nobel Prize in 2008.<ref name="Haldar" /> In the time since the work of these three researchers, GFP has been successfully expressed and utilized in bacteria, yeast, slime mold, plants, drosophila fruit flies, zebra-fish, and mammalian cells.<ref name="Yang" /> Below, mice have had GFP inserted into their genomes for studies in neurology. | ||
| Line 20: | Line 26: | ||
Green fluorescent protein (<scene name='Green_Fluorescent_Protein/1ema_gfp_default/2'>default scene</scene>) is a 21 kDa protein consisting of 238 residues strung together<ref>Primary structure at [http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=1ema&template=protein.html&r=wiring&l=1&chain=A www.ebi.aci.uk].</ref> to form a | Green fluorescent protein (<scene name='Green_Fluorescent_Protein/1ema_gfp_default/2'>default scene</scene>) is a 21 kDa protein consisting of 238 residues strung together<ref>Primary structure at [http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=1ema&template=protein.html&r=wiring&l=1&chain=A www.ebi.aci.uk].</ref> to form a | ||
| - | <scene name='Green_Fluorescent_Protein/1ema_gfp_barrel/2'>secondary structure</scene> of five α-helices and one eleven-stranded β-pleated sheet,<ref name="PDBsum" /> where each strand contains nine to thirteen residues each.<ref name="Ormo" /> (To view the primary and secondary structure of GFP, go to .) These β-strands display an almost “seamless symmetry” in which only two of the strands vary in structural content.<ref name="Phillips">PMID: 9434902</ref> This β-sheet conforms itself through regular hydrogen bonding into a β-barrel.<ref name="Yang" /> In GFP, the structure is so regular that <scene name='Green_Fluorescent_Protein/Water_stripes/1'>"stripes"</scene> of water molecules (red) can be seen following the structure of the barrel.<ref name="Phillips" /> Together with the α-helices at either end of the molecule, a nearly perfect cylinder is produced, 42Å long and 24Å in diameter,<ref name="Ormo" /> creating what is referred to as a “β-can” formation.<ref name="Phillips" /> The short helical segments at either end of the cylinder form “caps” to further protect the interior of the β-barrel.<ref name="Phillips" /> Overall stability is maintained by this β-can structure, helping to resist unfolding from heat and other denaturants.<ref name="Yang" /> | + | <scene name='Green_Fluorescent_Protein/1ema_gfp_barrel/2'>secondary structure</scene> of five α-helices and one eleven-stranded β-pleated sheet,<ref name="PDBsum" /> where each strand contains nine to thirteen residues each.<ref name="Ormo" /> (To view the primary and secondary structure of GFP, go to https://www.rcsb.org/pdb/explore/remediatedSequence.do?structureId=1EMA.) These β-strands display an almost “seamless symmetry” in which only two of the strands vary in structural content.<ref name="Phillips">PMID: 9434902</ref> This β-sheet conforms itself through regular hydrogen bonding into a β-barrel.<ref name="Yang" /> In GFP, the structure is so regular that <scene name='Green_Fluorescent_Protein/Water_stripes/1'>"stripes"</scene> of water molecules (red) can be seen following the structure of the barrel.<ref name="Phillips" /> Together with the α-helices at either end of the molecule, a nearly perfect cylinder is produced, 42Å long and 24Å in diameter,<ref name="Ormo" /> creating what is referred to as a “β-can” formation.<ref name="Phillips" /> The short helical segments at either end of the cylinder form “caps” to further protect the interior of the β-barrel.<ref name="Phillips" /> Overall stability is maintained by this β-can structure, helping to resist unfolding from heat and other denaturants.<ref name="Yang" /> |
| - | One < | + | One <jmol><jmolLink><script>script "/scripts/Green_Fluorescent_Protein/Central_helix/1.spt"; ppdiaCaptionCmd = "changeCaption('The central helix (shown in red) contains the fluorophore and runs through the barrel (shown as white transparent strands) along its axis (PDB-ID [[1ema]]). ','white','black');";javascript @ppdiaCaptionCmd;model 2;</script><text>α-helix</text></jmolLink></jmol> can be found running through the central axis of the β-barrel,<ref name="Haldar" /> roughly <scene name='Green_Fluorescent_Protein/Perpendicular/1'>perpendicular</scene> to the symmetry axis of the barrel.<ref name="Ormo">Ormo M, Cubitt AB, Kallio K, Gross LA, Tsien RY, Remington SJ. 1996. Crystal structure of the ''Aequorea victoria'' green fluorescent protein. Science. 273(5280):1392-1395. DOI 10.1126/science.273.5280.1392.</ref> This helix is extremely important as it contains the fluorophore responsible for fluorescence.<ref name="Yang" /><ref name="Haldar" /> |
| + | |||
| + | The fluorophore is part of the polypeptide chain (i.e. covalently connencted). If you press the buttom below, it will show the connection. | ||
| + | <jmol> | ||
| + | <jmolButton> | ||
| + | <script>select (64.C or 66.N1 or 66.C3 or 68.N); connect 1.1 1.6; select 64, 66, 68; wireframe 0.2; </script> | ||
| + | <text>make bonds</text> | ||
| + | </jmolButton> | ||
| + | </jmol> | ||
| + | |||
| + | This α-helix in particular is highly stabilized by the many <scene name='10/100139/Spacefill/1'>hydrophobic contacts</scene> that are made with each strand of the barrel.<ref name="Andrews">PMID:18713871</ref> | ||
| - | {{Link Toggle FancyCartoonHighQualityView}}. | ||
===The Chromophore=== | ===The Chromophore=== | ||
| Line 30: | Line 45: | ||
The <scene name='10/100139/Chromophore/2'>chromophore</scene> (<scene name='10/100139/Green_fluorescent_protein/1'>top view</scene>) of GFP is located at the center of the β-barrel with a wild-type excitation peak of 395 nm, and a minor peak at 475 nm (about three times less intense<ref name="Tsien" />) <ref name="Yang" /><ref name="Cubitt" /><ref name="Ormo" /><ref name="Phillips" /> with extinction coefficients of approximately 30,000 and 7,000 M<sup>-1</sup> cm<sup>-1</sup>, respectively.<ref name="Yang" /><ref name="Phillips" /> Interestingly, the ''Aequorea victoria'' jellyfish utilizes the smaller of the two excitation peaks as pure aequorin emits a light of 470 nm.<ref name="Tsien">Tsien, Roger Y. 1998. The Green Fluorescent Protein. Annual Review in Biochemistry. 67:509-544.</ref> The relative amplitudes of these two excitation peaks can vary depending on environmental factors and previous illumination.<ref name="Ormo" /> For example, continued excitation leads to a diminution of the 395 nm excitation peak with a reciprocal amplification of the 475 nm peak.<ref name="Phillips" /> Regardless of absorption, the chromophore of GFP emits light of 508 nm.<ref name="Yang" /><ref name="Cubitt" /><ref name="Ormo" /><ref name="Phillips" /> | The <scene name='10/100139/Chromophore/2'>chromophore</scene> (<scene name='10/100139/Green_fluorescent_protein/1'>top view</scene>) of GFP is located at the center of the β-barrel with a wild-type excitation peak of 395 nm, and a minor peak at 475 nm (about three times less intense<ref name="Tsien" />) <ref name="Yang" /><ref name="Cubitt" /><ref name="Ormo" /><ref name="Phillips" /> with extinction coefficients of approximately 30,000 and 7,000 M<sup>-1</sup> cm<sup>-1</sup>, respectively.<ref name="Yang" /><ref name="Phillips" /> Interestingly, the ''Aequorea victoria'' jellyfish utilizes the smaller of the two excitation peaks as pure aequorin emits a light of 470 nm.<ref name="Tsien">Tsien, Roger Y. 1998. The Green Fluorescent Protein. Annual Review in Biochemistry. 67:509-544.</ref> The relative amplitudes of these two excitation peaks can vary depending on environmental factors and previous illumination.<ref name="Ormo" /> For example, continued excitation leads to a diminution of the 395 nm excitation peak with a reciprocal amplification of the 475 nm peak.<ref name="Phillips" /> Regardless of absorption, the chromophore of GFP emits light of 508 nm.<ref name="Yang" /><ref name="Cubitt" /><ref name="Ormo" /><ref name="Phillips" /> | ||
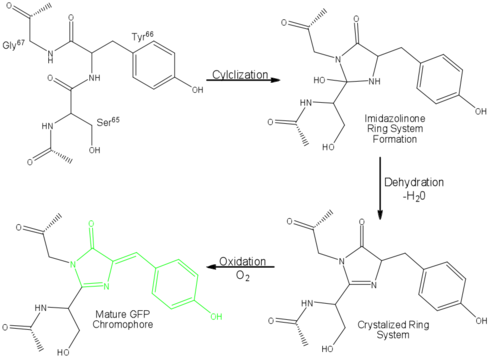
| - | Three amino residues in the central α-helix constitute the fluorophore of GFP: Ser<sup>65</sup>Tyr<sup>66</sup>Gly<sup>67</sup> (see below). Tsien et al. discovered that this tri-peptide sequence is post-translationally modified by internal cyclization and oxidation<ref name="Haldar" /> to produce a <scene name=' | + | Three amino residues in the central α-helix constitute the fluorophore of GFP: Ser<sup>65</sup>Tyr<sup>66</sup>Gly<sup>67</sup> (see below) or of EGFP: of GFP: Thr<sup>65</sup>Tyr<sup>66</sup>Gly<sup>67</sup>. Tsien et al. discovered that this tri-peptide sequence is post-translationally modified by internal cyclization and oxidation<ref name="Haldar" /> to produce a <scene name='10/100139/Chromophore_structure/3'>4-(p-hydroxybenzylidene)-imidazolidin-5-one</scene> structure (highlight atoms from <jmol><jmolLink><script> select (*.C1, *.CA1, *.N1, *.CB1, *.CG1, *.OG1) and 66; selectionHalos ON; delay 1.5;selectionHalos OFF;</script><text>⚞Thr 65⚟</text></jmolLink> </jmol>, <jmol><jmolLink><script> select (*.C2, *.O2, *.CA2, *.N2, *.CB2, *.CG2, *.CD1, *.CD2, *.CE1, *.CE2, *.CZ, *.OH) and 66; selectionHalos ON; delay 1.5;selectionHalos OFF;</script><text>⚞Tyr 66⚟</text></jmolLink> </jmol>, <jmol><jmolLink><script> select (*.O3,*.C3,*.CA3,*.N3) and 66; selectionHalos ON; delay 1.5;selectionHalos OFF;</script><text>⚞Gly 67⚟</text></jmolLink></jmol>).<ref name="Yang" /> Studies with E. coli proposed a sequential mechanism for the formation of the fluorophore that was initiated by a rapid cyclization between Ser<sup>65</sup> and Gly<sup>67</sup> to form an imidazolin-5-one intermediate.<ref name="Yang" /> This rapid cyclization is carried out via nucleophilic attack of the amino group from Gly<sup>67</sup> on the carbonyl group of Ser<sup>65</sup> to form a five-membered ring. The loss of water then forms the imidazolin-5-one intermediate.<ref name="Cubitt" /> Cyclization is succeeded by a much slower rate-limiting oxygenation of the Tyr<sup>66</sup> hydroxybenzyl side chain by atmospheric oxygen (No fluorescence was seen in anaerobically grown E. coli.), resulting in the 4-(p-hydroxybenzylidene)-imidazolidin-5-one stucture.<ref name="Yang" /><ref name="Cubitt" /><ref name="Phillips" /> The double bond that results from this series of reactions results in the linkage of the two π-systems of the rings, forming a <scene name='10/100139/Chromophore/5'>larger conjugated system</scene> essential for fluorophore stability. <ref name="Bublitz"> Bublitz G, King BA, Boxer SG. 1998. Electronic structure of the chromophore in green fluorescent protein (GFP). Journal of the American Chemical Society. 120(36): 9370-9371. DOI 10.1021/ja98160e.</ref> |
[[Image:GFP Chromophore.png|center|489x360px]] | [[Image:GFP Chromophore.png|center|489x360px]] | ||
The process is completely auto-catalytic such that there are no known co-factors or enzymatic components required.<ref name="Yang" /> Despite the stability of the final product, while the chromophore is forming, the environmental temperature cannot drop below 30°C or the yield of viable GFP will decrease substantially.<ref name="Yang" /><ref name="Phillips" /> This, of course, is not an issue for the protein in nature as the jellyfish is unlikely to encounter waters of this degree in the Pacific Northwest.<ref name="Tsien" /> Such a temperature sensitivity is only relevant during formation as the stability of the final product is maintained through a network of close contacts surrounding the fluorophore.<ref name="Yang" /> This, however, can and has been used in [[pulse-chase experiments]] in which the GFP-expressing cells are exposed to varying temperatures in place of labeled vs. unlabeled trials.<ref name="Tsien" /> | The process is completely auto-catalytic such that there are no known co-factors or enzymatic components required.<ref name="Yang" /> Despite the stability of the final product, while the chromophore is forming, the environmental temperature cannot drop below 30°C or the yield of viable GFP will decrease substantially.<ref name="Yang" /><ref name="Phillips" /> This, of course, is not an issue for the protein in nature as the jellyfish is unlikely to encounter waters of this degree in the Pacific Northwest.<ref name="Tsien" /> Such a temperature sensitivity is only relevant during formation as the stability of the final product is maintained through a network of close contacts surrounding the fluorophore.<ref name="Yang" /> This, however, can and has been used in [[pulse-chase experiments]] in which the GFP-expressing cells are exposed to varying temperatures in place of labeled vs. unlabeled trials.<ref name="Tsien" /> | ||
| - | As the central α-helix is not located directly in the center of the β-barrel, cavities of differing area exist on either side of the chromophore. The larger cavity, consisting of about 135 Å,<ref name="Ormo" /> does not open out to the bulk solvent, but rather houses <scene name='Green_Fluorescent_Protein/Water_molecules/1'>four water molecules</scene>.<ref name="Ormo" /><ref name="Van">van Thor JJ, Sage, JT. 2006. Charge transfer in green fluorescent protein. Photochemical & Photobiological Sciences. 5:597-602. DOI 10.1039/b516525c.</ref> Had this space not been occupied, it would be expected to considerably destabilize the protein as a whole. The hydrogen bonding created by the presence of the water molecules, however, helps to link the buried <scene name='Green_Fluorescent_Protein/Gln69_glu222/1 | + | As the central α-helix is not located directly in the center of the β-barrel, cavities of differing area exist on either side of the chromophore. The larger cavity, consisting of about 135 cubic Å,<ref name="Ormo" /> does not open out to the bulk solvent, but rather houses <scene name='Green_Fluorescent_Protein/Water_molecules/1'>four water molecules</scene>.<ref name="Ormo" /><ref name="Van">van Thor JJ, Sage, JT. 2006. Charge transfer in green fluorescent protein. Photochemical & Photobiological Sciences. 5:597-602. DOI 10.1039/b516525c.</ref> Had this space not been occupied, it would be expected to considerably destabilize the protein as a whole. The hydrogen bonding created by the presence of the water molecules, however, helps to link the buried <scene name='Green_Fluorescent_Protein/Gln69_glu222/1'>side chains</scene> of Glu<sup>222</sup> and Gln<sup>69</sup> that would otherwise be actively polar.<ref name="Ormo" /> Therefore, the water molecules are extremely important in establishing a hydrogen bonding network about the chromophor.<ref name="Lammich">PMID: 17040991</ref> |
| - | The opposite side of the chromophore, however, is within close proximity of several aromatic and polar side chains. Several <scene name='Green_Fluorescent_Protein/Polar_interactions/2'>polar interactions</scene> between the surrounding residues and the chromophore are present including: hydrogen bonds of His<sup>148</sup>, Thr<sup>203</sup>, and Ser<sup>205</sup> with the phenolic hydroxyl of Tyr<sup>66</sup>; Arg<sup>96</sup> and Gln<sup>94</sup> with the carbonyl of the imidazolidinone ring; and hydrogen bonds of Glu<sup>222</sup> with the side chain of Thr<sup>65</sup>. Additional hydrogen bonding in the area around the chromophore helps to stabilize Arg<sup>96</sup> in the protonated form, which suggests the presence of a partial negative charge on the carbonyl oxygen of the imidazolidinone ring in the deprotonated fluorophore.<ref name="Ormo" /> Arg<sup>96</sup> and Gln<sup>94</sup> in turn help to steady the imidazolidone.<ref name="Yang" /> Therefore, it is thought that Arg<sup>96</sup> is essential for the formation of the fluorophore by catalyzing the initial ring closure.<ref name="Ormo" /> Tyr<sup>145</sup> provides a stabilizing | + | The opposite side of the chromophore, however, is within close proximity of several aromatic and polar side chains. Several <scene name='Green_Fluorescent_Protein/Polar_interactions/2'>polar interactions</scene> between the surrounding residues and the chromophore are present including: hydrogen bonds of His<sup>148</sup>, Thr<sup>203</sup>, and Ser<sup>205</sup> with the phenolic hydroxyl of Tyr<sup>66</sup>; Arg<sup>96</sup> and Gln<sup>94</sup> with the carbonyl of the imidazolidinone ring; and hydrogen bonds of Glu<sup>222</sup> with the side chain of Thr<sup>65</sup>. Additional hydrogen bonding in the area around the chromophore helps to stabilize Arg<sup>96</sup> in the protonated form, which suggests the presence of a partial negative charge on the carbonyl oxygen of the imidazolidinone ring in the deprotonated fluorophore.<ref name="Ormo" /> Arg<sup>96</sup> and Gln<sup>94</sup> in turn help to steady the imidazolidone.<ref name="Yang" /> Therefore, it is thought that Arg<sup>96</sup> is essential for the formation of the fluorophore by catalyzing the initial ring closure.<ref name="Ormo" /> Tyr<sup>145</sup> provides a stabilizing <scene name='Green_Fluorescent_Protein/Edge_face_interaction/1'>edge-face interaction</scene><ref> [http://www.tim.hi-ho.ne.jp/dionisio/ Information about edge-face (CH/π) interactions].</ref> with the benzyl ring of the chromophore.<ref name="Ormo" /> The stability provided by the internal polar interactions are further augmented by the surrounding β-barrel. |
| - | <scene name='Green_Fluorescent_Protein/Edge_face_interaction/1'>edge-face interaction</scene><ref> [http://www.tim.hi-ho.ne.jp/dionisio/ Information about edge-face (CH/π) interactions].</ref> with the benzyl ring of the chromophore.<ref name="Ormo" /> The stability provided by the internal polar interactions are further augmented by the surrounding β-barrel. | + | |
The β-barrel provides a highly constrained environment that protects the chromophore from the bulk solvent,<ref name="Haldar" /> nearly creating the atmosphere of a vacuum.<ref name="Lammich" /> This is most likely responsible for the small [[Stoke’s shift]], or the small wavelength difference between excitation and emission.<ref name="Ormo" /> | The β-barrel provides a highly constrained environment that protects the chromophore from the bulk solvent,<ref name="Haldar" /> nearly creating the atmosphere of a vacuum.<ref name="Lammich" /> This is most likely responsible for the small [[Stoke’s shift]], or the small wavelength difference between excitation and emission.<ref name="Ormo" /> | ||
| Line 46: | Line 60: | ||
Findings show that fluorescence will not occur from a naked chromophore, but rather requires the protection of the β-can structure.<ref name="Cubitt" /> However, ''in crystallum'' GFP will exhibit a nearly identical fluorescence spectrum and lifetime when compared with aqueous GFP. These two elements point to a fluorescence that is not inherent to the isolated fluorophore,<ref name="Yang" /><ref name="Phillips" /> but rather from the auto-catalytic cyclization of the polypeptide sequence Ser<sup>65</sup>Tyr<sup>66</sup>Gly<sup>67</sup> and subsequent oxidation of Tyr<sup>66</sup>.<ref name="Phillips" /> However, this sequence is found in many proteins - why does GFP fluoresce? According to Phillips (1997), fluorophore formation is due to the close proximity of the backbone atoms between Ser<sup>65</sup>. and Gly<sup>67</sup> gained through a lack of sterical hindrance by the hydrogen atom side chain of glycine. In fact, no functional fluorescent proteins have been found in which any other amino acid other than glycine was found at position 67. Even so, there are still proteins that have this specific sequence, therefore, there must be another inherent property to GFP that is still left misunderstood.<ref name="Phillips" /> | Findings show that fluorescence will not occur from a naked chromophore, but rather requires the protection of the β-can structure.<ref name="Cubitt" /> However, ''in crystallum'' GFP will exhibit a nearly identical fluorescence spectrum and lifetime when compared with aqueous GFP. These two elements point to a fluorescence that is not inherent to the isolated fluorophore,<ref name="Yang" /><ref name="Phillips" /> but rather from the auto-catalytic cyclization of the polypeptide sequence Ser<sup>65</sup>Tyr<sup>66</sup>Gly<sup>67</sup> and subsequent oxidation of Tyr<sup>66</sup>.<ref name="Phillips" /> However, this sequence is found in many proteins - why does GFP fluoresce? According to Phillips (1997), fluorophore formation is due to the close proximity of the backbone atoms between Ser<sup>65</sup>. and Gly<sup>67</sup> gained through a lack of sterical hindrance by the hydrogen atom side chain of glycine. In fact, no functional fluorescent proteins have been found in which any other amino acid other than glycine was found at position 67. Even so, there are still proteins that have this specific sequence, therefore, there must be another inherent property to GFP that is still left misunderstood.<ref name="Phillips" /> | ||
| - | This quandary led Phillips to study the acid/base chemistry catalyzing the initial cyclization of the chromophore. He found that Arg<sup>96</sup> actually acts as a | + | This quandary led Phillips to study the acid/base chemistry catalyzing the initial cyclization of the chromophore. He found that Arg<sup>96</sup> actually acts as a <scene name='Green_Fluorescent_Protein/Arg96/1' >base</scene> by withdrawing electrons through hydrogen bonding with the carbonyl oxygen of Ser<sup>65</sup> to activate the carbonyl carbon for nucleophilic attack by the amide nitrogen of Gly<sup>67</sup>. This mechanism was further supported by ''ab initio'' calculations, as well as database searches of similar compounds and protein sequences. Through acid/base chemistry, the chromophore is stabilized by resonance.<ref name="Phillips" /> Femtosecond Raman spectroscopy has been used to map the alteration of the structure close the chromophore during excited-state protein transfer and shown that chromophore wagging is orchestrated by the protein environment.<ref name="Fang">PMID: 19907490</ref> |
| - | <scene name='Green_Fluorescent_Protein/Arg96/1 | + | |
===Mutant Studies=== | ===Mutant Studies=== | ||
Many mutant green fluorescent proteins have been developed in order to further understand the structure and mechanism of the fluorophore. The first mutagenesis studies simply | Many mutant green fluorescent proteins have been developed in order to further understand the structure and mechanism of the fluorophore. The first mutagenesis studies simply | ||
| - | <scene name='Green_Fluorescent_Protein/Truncated_ends/3 | + | <scene name='Green_Fluorescent_Protein/Truncated_ends/3' >truncated the ends</scene> of the amino acid sequence (<scene name='Green_Fluorescent_Protein/1ema_gfp_barrel/2' >see without truncated ends</scene>. NOTE: The structure represented here is already truncated at the carbonyl terminus). Shortening the polypeptide by more than seven amino acids from either terminus lead to a total loss of fluorescence, as well as a complete failure to absorb light at the traditional wavelengths. This is most likely due to the structure of the protein. The last seven amino acid residues of the carboxyl terminus are roughly disordered, and thus do not interfere with the overall structure. After seven residues, however, the capping α-helix structure is disrupted, leading to an unstable or unformed chromophore. The <scene name='Green_Fluorescent_Protein/Amino_terminus/2'>amino terminus</scene> is less understood, but the same principle still applies even though the β-barrel does not begin until residue ten or eleven.<ref name="Yang" /> |
Point mutations have also been extensively studied in order to examine their effects on the chromophore. In general, most point mutations lead to a diminished excitation, especially in regions of the sequence adjacent to the fluorophore or those that interact with the fluorophore. An exception to this trend is the Ser<sup>65</sup>Thr<sup>66</sup> mutant (normal Ser<sup>65</sup>Tyr<sup>66</sup>), which actually increases fluorescence intensity, although the reason is unclear.<ref name="Yang" /> | Point mutations have also been extensively studied in order to examine their effects on the chromophore. In general, most point mutations lead to a diminished excitation, especially in regions of the sequence adjacent to the fluorophore or those that interact with the fluorophore. An exception to this trend is the Ser<sup>65</sup>Thr<sup>66</sup> mutant (normal Ser<sup>65</sup>Tyr<sup>66</sup>), which actually increases fluorescence intensity, although the reason is unclear.<ref name="Yang" /> | ||
| Line 58: | Line 71: | ||
An interesting mutation discovered by Ormo et al. (1996) was the Thr<sup>65</sup>Tyr<sup>66</sup>Gly<sup>67</sup> mutant, which produces an α-helical conformation in the chromophore opposed to the normal conformation, which is nearly perpendicular to the helical axis, due to its interaction with Arg<sup>96</sup>. This further supports the idea that Arg<sup>96</sup> is an important factor in the structural arrangement required for cyclization, perhaps by promoting the attack of Gly<sup>67</sup> on the carbonyl carbon of Thr<sup>65</sup>.<ref name="Ormo" /> | An interesting mutation discovered by Ormo et al. (1996) was the Thr<sup>65</sup>Tyr<sup>66</sup>Gly<sup>67</sup> mutant, which produces an α-helical conformation in the chromophore opposed to the normal conformation, which is nearly perpendicular to the helical axis, due to its interaction with Arg<sup>96</sup>. This further supports the idea that Arg<sup>96</sup> is an important factor in the structural arrangement required for cyclization, perhaps by promoting the attack of Gly<sup>67</sup> on the carbonyl carbon of Thr<sup>65</sup>.<ref name="Ormo" /> | ||
| - | In high protein concentrations, GFP has been found to dimerize under the influence of high ionic strength between the two monomers. In ''Aequorea victoria'', the aequorin is able to bind to the <scene name='Green_Fluorescent_Protein/1gfl/1 | + | In high protein concentrations, GFP has been found to dimerize under the influence of high ionic strength between the two monomers. In ''Aequorea victoria'', the aequorin is able to bind to the <scene name='Green_Fluorescent_Protein/1gfl/1' >dimer</scene> ([[1gfl]]), but not the monomer. Therefore, dimerization is a very important structural feature in terms of its function, as it also assists the GFP to absorb energy at the excitation wavelength of aequorin even though GFP has only a “modest” extinction coefficient. As a result, dimers, and often even higher <scene name='Green_Fluorescent_Protein/1w7s/1'>multimers</scene> ([[1w7s]]), are predominant protein populations within the jellyfish.<ref name="Cubitt">[http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6TCV-40W0TN7-50&_user=4187488&_coverDate=11%2F30%2F1995&_rdoc=1&_fmt=high&_orig=search&_sort=d&_docanchor=&view=c&_acct=C000062504&_version=1&_urlVersion=0&_userid=4187488&md5=e92730038bb92b1dfbd4af45a0283cce],Cubitt AB, Heim R, Adams SR, Boyd AE, Gross LA, Tsien R. 1995. Understanding, improving, and using green fluorescent protein. Trends in Biochemical Sciences. 20(11): 448-455. DOI 0.1016/S0968-0004(00)89099-4.</ref> |
{{Link Toggle FancyCartoonHighQualityView}}. | {{Link Toggle FancyCartoonHighQualityView}}. | ||
| Line 66: | Line 79: | ||
A description of some of the ways GFP is being used as a tool in research is at [[Green_Fluorescent_Protein:_Research_Tool]]. | A description of some of the ways GFP is being used as a tool in research is at [[Green_Fluorescent_Protein:_Research_Tool]]. | ||
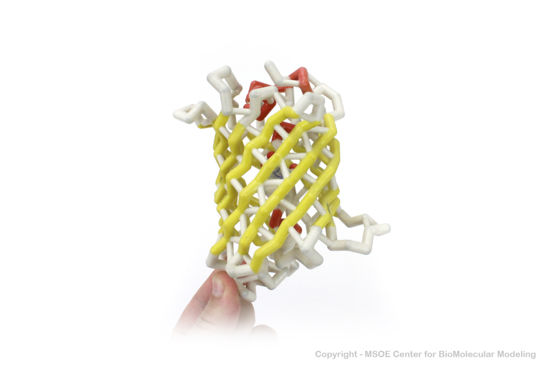
| - | + | ==3D Printed Physical Model of Green Fluorescent Protein (GFP)== | |
| + | Shown below are 3D printed physical models of the Green Fluorescent Protein (GFP). The first alpha carbon backbone model is colored by three-strand repeats, including red, blue, purple, and yellow. The second alpha carbon backbone model is colored by secondary structure, with alpha helices red and beta sheets yellow. Both models show the fluorophore molecule at the center of the GFP structure. | ||
| - | + | [[Image:gfp1_centerForBioMolecularModeling.jpg | 550px]] | |
| - | + | [[Image:gfp2_centerForBioMolecularModeling.jpg | 550px]] | |
| - | + | ====The MSOE Center for BioMolecular Modeling==== | |
| - | + | [[Image:CbmUniversityLogo.jpg | left | 150px]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | The [http://cbm.msoe.edu MSOE Center for BioMolecular Modeling] uses 3D printing technology to create physical models of protein and molecular structures, making the invisible molecular world more tangible and comprehensible. To view more protein structure models, visit our [http://cbm.msoe.edu/educationalmedia/modelgallery/ Model Gallery]. | |
| - | ** [[3dpw]], [[3dpx]], [[3dpz]], [[3dq1]], [[3dq2]], [[3dq3]], [[3dq4]], [[3dq5]], [[3dq6]], [[3dq7]], [[3dq8]], [[3dq9]], [[3dqa]], [[3dqc]], [[3dqd]], [[3dqe]], [[3dqf]], [[3dqh]], [[3dqi]], [[3dqj]], [[3dqk]], [[3dql]], [[3dqm]], [[3dqn]], [[3dqo]], [[3dqu]], [[1myw]], [[1huy]], [[2yfp]], [[1yfp]], [[3ed8]], [[3v3d]] – jGFP (mutant)<br /> | ||
| - | ** [[1f09]], [[1f0b]] – jGFP (mutant)+imidazole derivative+I<br /> | ||
| - | ** [[2ogr]] – Z-FP - Zoanthus<br /> | ||
| - | ** [[2pxs]], [[2pxw]], [[1xa9]], [[1xae]] – Z-FP (mutant)<br /> | ||
| - | ** [[2jad]] – jGFP/glutaredoxin<br /> | ||
| - | * Red fluorescent protein | ||
| - | + | ==3D structures of Green Fluorescent Protein == | |
| - | + | [[Green Fluorescent Protein 3D structures]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | </StructureSection> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==Reference for this Structure== | ==Reference for this Structure== | ||
| Line 196: | Line 124: | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
| + | [[Category:3D printer files]] | ||
Current revision
| |||||||||||
Reference for this Structure
Ormo M, Cubitt AB, Kallio K, Gross LA, Tsien RY, Remington SJ. 1996. Crystal structure of the Aequorea victoria green fluorescent protein. Science. 273(5280):1392-1395. DOI 10.1126/science.273.5280.1392.
References
- ↑ 1.0 1.1 [1], Protein Database (PDBsum): 1ema. European Bioinformatics (EBI); 2009.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 [2], Yang F, Moss LG, Phillips GN Jr. 1996. The molecular structure of green fluorescent protein. Biotechnology. 14: 1246-1251. DOI 10.1038/nbt1096-1246.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Tsien, Roger Y. 1998. The Green Fluorescent Protein. Annual Review in Biochemistry. 67:509-544.
- ↑ Bek JW, De Clercq A, De Saffel H, Soenens M, Huysseune A, Witten PE, Coucke PJ, Willaert A. Photoconvertible fluorescent proteins: a versatile tool in zebrafish skeletal imaging. J Fish Biol. 2021 Apr;98(4):1007-1017. PMID:32242924 doi:10.1111/jfb.14335
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 [3], Haldar S, Chattopadhyay A. 2009. The green journey. J Fluoresc. 19:1-2. DOI 10.1007/s10895-008-0455-6; biographical background on Douglas Prasher, Martin Chalfie and Roger Tsien.
- ↑ 6.0 6.1 6.2 6.3 [4], Shimomura O. The discovery of green fluorescent protein. Nobel Prize Lecture; 2009;; biographical background at Wikipedia.
- ↑ [5],Cowles D, Cowles J. Aequorea victoria. 2007. Walla Wall University.
- ↑ Primary structure at www.ebi.aci.uk.
- ↑ 9.00 9.01 9.02 9.03 9.04 9.05 9.06 9.07 9.08 9.09 9.10 9.11 9.12 9.13 Ormo M, Cubitt AB, Kallio K, Gross LA, Tsien RY, Remington SJ. 1996. Crystal structure of the Aequorea victoria green fluorescent protein. Science. 273(5280):1392-1395. DOI 10.1126/science.273.5280.1392.
- ↑ 10.00 10.01 10.02 10.03 10.04 10.05 10.06 10.07 10.08 10.09 10.10 10.11 10.12 10.13 Phillips GN Jr. Structure and dynamics of green fluorescent protein. Curr Opin Struct Biol. 1997 Dec;7(6):821-7. PMID:9434902
- ↑ Andrews BT, Gosavi S, Finke JM, Onuchic JN, Jennings PA. The dual-basin landscape in GFP folding. Proc Natl Acad Sci U S A. 2008 Aug 26;105(34):12283-8. Epub 2008 Aug 19. PMID:18713871
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 [6],Cubitt AB, Heim R, Adams SR, Boyd AE, Gross LA, Tsien R. 1995. Understanding, improving, and using green fluorescent protein. Trends in Biochemical Sciences. 20(11): 448-455. DOI 0.1016/S0968-0004(00)89099-4.
- ↑ Bublitz G, King BA, Boxer SG. 1998. Electronic structure of the chromophore in green fluorescent protein (GFP). Journal of the American Chemical Society. 120(36): 9370-9371. DOI 10.1021/ja98160e.
- ↑ van Thor JJ, Sage, JT. 2006. Charge transfer in green fluorescent protein. Photochemical & Photobiological Sciences. 5:597-602. DOI 10.1039/b516525c.
- ↑ 15.0 15.1 Lammich L, Petersen MA, Nielsen MB, Andersen LH. The gas-phase absorption spectrum of a neutral GFP model chromophore. Biophys J. 2007 Jan 1;92(1):201-7. Epub 2006 Oct 13. PMID:17040991 doi:10.1529/biophysj.106.093674
- ↑ Information about edge-face (CH/π) interactions.
- ↑ Fang C, Frontiera RR, Tran R, Mathies RA. Mapping GFP structure evolution during proton transfer with femtosecond Raman spectroscopy. Nature. 2009 Nov 12;462(7270):200-4. PMID:19907490 doi:10.1038/nature08527
Additional Resources
- For additional information, see: Colored & Bioluminescent Proteins
- First Glance
- PDBsum: 1ema
- RCSB PDB 1ema
- OCA
- UniProt: P42212
- Scop: P42212
- CATH: 1emaA00
- Pfam: PF01353
- InterPro: IPR000786
- GFP featured at the Molecule of the Month series of tutorials by David Goodsell.
- Inside green fluorescent protein - editor's summary that accompanied structural detail of GFP chromophore on the cover of Nature.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Wayne Decatur, Karsten Theis, Eran Hodis, Laura Carbone, Karl Oberholser, Mark Hoelzer, Joel L. Sussman, Alexander Berchansky, Jaime Prilusky, Marius Mihasan, Joseph M. Steinberger, David Canner