|
|
| (12 intermediate revisions not shown.) |
| Line 1: |
Line 1: |
| - | {{STRUCTURE_3ouh| PDB=3ouh | SIZE=400| SCENE= |right|CAPTION=Human PHD2 catalytic domain complex with Fe+2 ion (orange), inhibitor and sulfate, [[3ouh]] }}
| + | <StructureSection load='' size='400' side='right' scene='45/459221/Cv/1' caption='Human PHD2 catalytic domain complex with Fe+2 ion (orange), inhibitor and sulfate, [[3ouh]]' pspeed='8'> |
| - |
| + | |
| - | '''Prolyl hydroxylase domain''' (PHD) proteins mediate oxygen-dependent degradation of Hypoxia-inducible factor (HIF) α subunit. They include PHD1, PHD2 and PHD3. The PHD is a Fe+2/oxogluterate (2OG)-dependent enzyme. [[3ouh]] is the crystallized structure of the enzyme PHD2, an [[oxidoreductase]] that is 237 amino acids long with a molecular weight of 27 kDa. [[3ouh]] is found in [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens] and is a homolog of [http://en.wikipedia.org/wiki/EGLN1 EGLN1] found in [http://en.wikipedia.org/wiki/Caenorhabditis_elegans C. elegans]. | + | |
| - | The protein has three ligands: O14 (a 1-(5-chloro-6-fluoro-1H-benzimidazol-2-yl)-1H-pyrazole-4-carboxylic acid), FE+2 (an iron ion), and SO<sub>4</sub> (a sulfate ion). It is involved in mediating physiological responses to [http://en.wikipedia.org/wiki/Hypoxia_(medical) hypoxia] by degrading the transcription factor of a hypoxia-inducible factor HIF1-α. In hypoxic conditions, the activity of PHD2 lessens, causing an increase in HIF1-α, resulting in secretion of erythropoietin, anaerobic [[glycolysis]], and angiogenesis<ref>PMID:16686427</ref>.
| + | |
| - | <ref>Rosen M D, Venkatesan H, Peltier H M, Bembenek S D, Kanelakis K C, Zhao L X, Leonard B E, Hocutt F M, Wu X, Palomino H L, Brondtetter T I, Haugh P V, Cagnon L, Yan W, Liotta L A, Young A, Mirzadegan T, Shankley N P, Barrett T D, Rabinowitz M H. Benzimidazole-2-pyrazole HIF Prolyl 4-Hydroxylase Inhibitors as Oral Erythropoietin Secretagogues. ACS Medicinal Chemical Letters. 2010 Oct 5.</ref> For more detalis see [[Molecular Playground/Prolyl Hydroxylase Domain (PHD) Enzyme]].
| + | |
| | | | |
| | + | See also [[Hydroxylase]] |
| | + | |
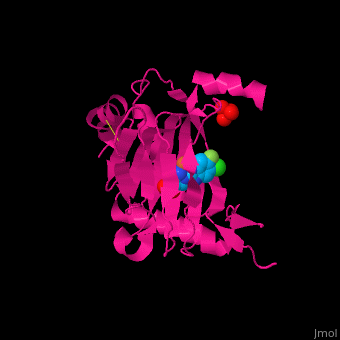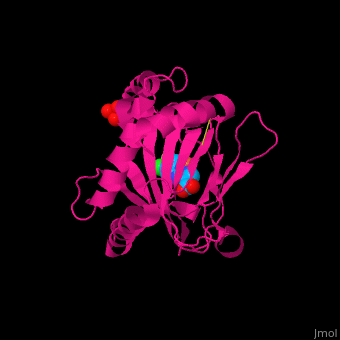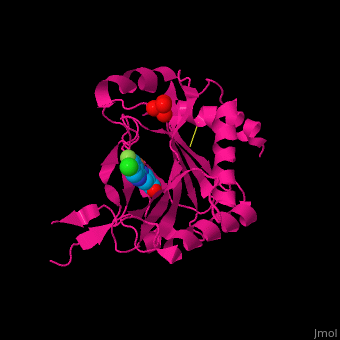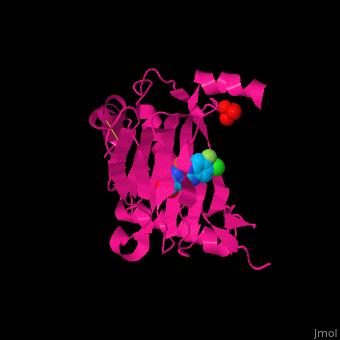
| | + | '''Prolyl hydroxylase domain''' (PHD) or '''egl nine homolog 1''' (PHD2/EGLN1) proteins mediate oxygen-dependent degradation of Hypoxia-inducible factor (HIF) α subunit. They include PHD1, PHD2 and PHD3. The PHD is a Fe+2/oxogluterate (2OG)-dependent enzyme. [[3ouh]] is the crystallized structure of the enzyme PHD2, an [[oxidoreductase]] that is 237 amino acids long with a molecular weight of 27 kDa. [[3ouh]] is found in [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens] and is a homolog of [http://en.wikipedia.org/wiki/EGLN1 EGLN1] found in [http://en.wikipedia.org/wiki/Caenorhabditis_elegans C. elegans]. |
| | + | The protein has three ligands: <scene name='45/459221/Cv/4'>O14</scene> (a 1-(5-chloro-6-fluoro-1H-benzimidazol-2-yl)-1H-pyrazole-4-carboxylic acid), <scene name='45/459221/Cv/3'>Fe+2 (an iron ion)</scene>, and SO<sub>4</sub> (a sulfate ion). Water molecules are shown as red spheres. It is involved in mediating physiological responses to [http://en.wikipedia.org/wiki/Hypoxia_(medical) hypoxia] by degrading the transcription factor of a hypoxia-inducible factor HIF1-α. In hypoxic conditions, the activity of PHD2 lessens, causing an increase in HIF1-α, resulting in secretion of erythropoietin, anaerobic [[glycolysis]], and angiogenesis<ref>PMID:16686427</ref>. |
| | + | <ref>Rosen M D, Venkatesan H, Peltier H M, Bembenek S D, Kanelakis K C, Zhao L X, Leonard B E, Hocutt F M, Wu X, Palomino H L, Brondtetter T I, Haugh P V, Cagnon L, Yan W, Liotta L A, Young A, Mirzadegan T, Shankley N P, Barrett T D, Rabinowitz M H. Benzimidazole-2-pyrazole HIF Prolyl 4-Hydroxylase Inhibitors as Oral Erythropoietin Secretagogues. ACS Medicinal Chemical Letters. 2010 Oct 5.</ref> For more detalis see [[Molecular Playground/Prolyl Hydroxylase Domain (PHD) Enzyme]]. |
| | | | |
| | == 3D Structures of prolyl hydroxylase domain == | | == 3D Structures of prolyl hydroxylase domain == |
| | | | |
| - | [[2y33]] – hPHD2 catalytic domain + Zn + chloro-iodoisoquinolin-carbonyl glycine – human<br /> | + | [[Polyl hydroxylase domain 3D structures]] |
| - | [[2y34]] - hPHD2 catalytic domain + Fe + chloro-iodoisoquinolin-carbonyl glycine<br />
| + | |
| - | [[3ouh]], [[3oui]] - hPHD2 catalytic domain + Fe + inhibitor<br />
| + | |
| - | [[3ouj]] - hPHD2 catalytic domain + Fe + 2OG<br />
| + | |
| - | [[3hqr]] - hPHD2 catalytic domain (mutant) + Mn + HIF 1 α C terminal + oxalylglycine<br />
| + | |
| - | [[3hqu]] - hPHD2 catalytic domain + Fe + HIF 1 α C terminal + chloro-iodoisoquinolin-carbonyl glycine<br />
| + | |
| - | [[2hbt]], [[2hbu]] - hPHD2 catalytic domain + Fe + chloro-iodoisoquinolin-carbonyl glycine<br />
| + | |
| - | [[2g19]], [[2g1m]] - hPHD2 catalytic domain + Fe + hydroxy-iodoisoquinolin-carbonyl glycine<br />
| + | |
| - | [[4h6j]] – hPHD Pasb domain (mutant) + aryl hydrocarbon nuclear translocator (mutant)<br />
| + | |
| | ==References== | | ==References== |
| | <references/> | | <references/> |
| | + | </StructureSection> |
| | [[Category:Topic Page]] | | [[Category:Topic Page]] |
| | <br /> | | <br /> |
| | Created with the participation of [[User:Andrew Winslow|Andrew Winslow]]. | | Created with the participation of [[User:Andrew Winslow|Andrew Winslow]]. |
|
See also Hydroxylase
Prolyl hydroxylase domain (PHD) or egl nine homolog 1 (PHD2/EGLN1) proteins mediate oxygen-dependent degradation of Hypoxia-inducible factor (HIF) α subunit. They include PHD1, PHD2 and PHD3. The PHD is a Fe+2/oxogluterate (2OG)-dependent enzyme. 3ouh is the crystallized structure of the enzyme PHD2, an oxidoreductase that is 237 amino acids long with a molecular weight of 27 kDa. 3ouh is found in Homo sapiens and is a homolog of EGLN1 found in C. elegans.
The protein has three ligands: (a 1-(5-chloro-6-fluoro-1H-benzimidazol-2-yl)-1H-pyrazole-4-carboxylic acid), , and SO4 (a sulfate ion). Water molecules are shown as red spheres. It is involved in mediating physiological responses to hypoxia by degrading the transcription factor of a hypoxia-inducible factor HIF1-α. In hypoxic conditions, the activity of PHD2 lessens, causing an increase in HIF1-α, resulting in secretion of erythropoietin, anaerobic glycolysis, and angiogenesis[1].
[2] For more detalis see Molecular Playground/Prolyl Hydroxylase Domain (PHD) Enzyme.
3D Structures of prolyl hydroxylase domain
Polyl hydroxylase domain 3D structures
References
- ↑ Stolze IP, Mole DR, Ratcliffe PJ. Regulation of HIF: prolyl hydroxylases. Novartis Found Symp. 2006;272:15-25; discussion 25-36. PMID:16686427
- ↑ Rosen M D, Venkatesan H, Peltier H M, Bembenek S D, Kanelakis K C, Zhao L X, Leonard B E, Hocutt F M, Wu X, Palomino H L, Brondtetter T I, Haugh P V, Cagnon L, Yan W, Liotta L A, Young A, Mirzadegan T, Shankley N P, Barrett T D, Rabinowitz M H. Benzimidazole-2-pyrazole HIF Prolyl 4-Hydroxylase Inhibitors as Oral Erythropoietin Secretagogues. ACS Medicinal Chemical Letters. 2010 Oct 5.
|