Protein kinase C
From Proteopedia
(Difference between revisions)
| (11 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
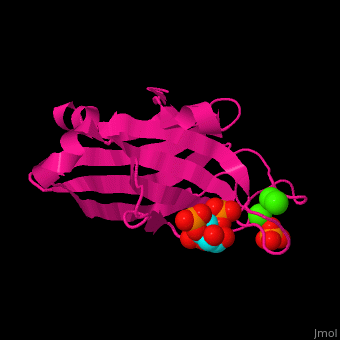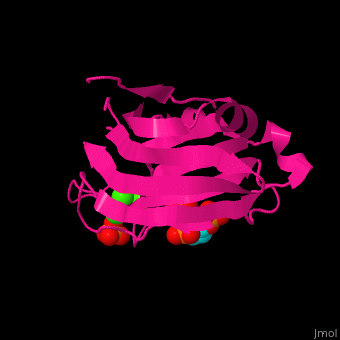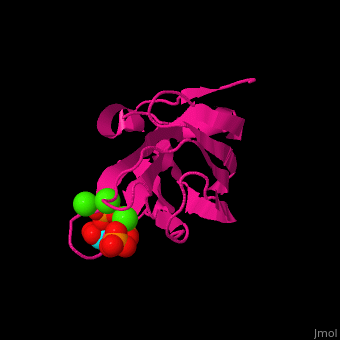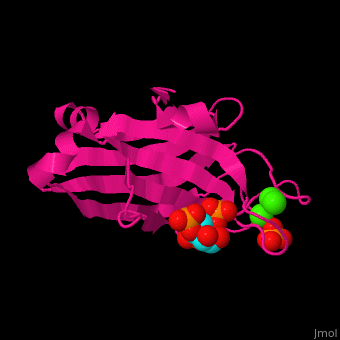
| - | <StructureSection load=' | + | <StructureSection load='' size='350' side='right' caption='Rat protein kinase α C2 domain complex with phosphatidylinositol, phosphate and Ca+2 ion (green), [[3gpe]] ' scene='46/468129/Cv/1'> |
| + | __TOC__ | ||
== Function == | == Function == | ||
'''Protein kinase C''' (PKC) phosphorylates serine or threonine residues in proteins. PKC act in signal transduction pathways<ref>PMID:11440359</ref>. PKC consists of regulatory domain hinged to a catalytic domain. The regulatory domain contains the C1 region which binds diacylglycerol (DAG) and phorbol esters and the C2 domain which is a Ca+2 sensor. PKC contains Pleckstrin Homology (PH) domain which binds phosphatidylinositol lipids (PTDINS). The PH domain is found in proteins involved in intracellular signaling. | '''Protein kinase C''' (PKC) phosphorylates serine or threonine residues in proteins. PKC act in signal transduction pathways<ref>PMID:11440359</ref>. PKC consists of regulatory domain hinged to a catalytic domain. The regulatory domain contains the C1 region which binds diacylglycerol (DAG) and phorbol esters and the C2 domain which is a Ca+2 sensor. PKC contains Pleckstrin Homology (PH) domain which binds phosphatidylinositol lipids (PTDINS). The PH domain is found in proteins involved in intracellular signaling. | ||
| Line 11: | Line 12: | ||
== Structural highlights == | == Structural highlights == | ||
| - | Phosphatidylinositol binds to PKC-α C2 domain groove near the Ca+2 binding pocket<ref>PMID:19346474</ref>. | + | <scene name='46/468129/Cv/6'>Phosphatidylinositol binds to PKC-α C2 domain groove</scene> near the <scene name='46/468129/Cv/7'>Ca+2 binding pocket</scene><ref>PMID:19346474</ref>. Water molecules are shown as red spheres. |
| - | + | ||
==3D structures of protein kinase C== | ==3D structures of protein kinase C== | ||
| + | [[Protein kinase C 3D structures]] | ||
| - | + | </StructureSection> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | **[[2d9z]] – hPKC-ν PH domain - NMR | ||
| - | }} | ||
== References == | == References == | ||
<references/> | <references/> | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
References
- ↑ Idris I, Gray S, Donnelly R. Protein kinase C activation: isozyme-specific effects on metabolism and cardiovascular complications in diabetes. Diabetologia. 2001 Jun;44(6):659-73. PMID:11440359 doi:http://dx.doi.org/10.1007/s001250051675
- ↑ Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes. 1998 Jun;47(6):859-66. PMID:9604860
- ↑ Koivunen J, Aaltonen V, Peltonen J. Protein kinase C (PKC) family in cancer progression. Cancer Lett. 2006 Apr 8;235(1):1-10. PMID:15907369 doi:http://dx.doi.org/10.1016/j.canlet.2005.03.033
- ↑ Guerrero-Valero M, Ferrer-Orta C, Querol-Audi J, Marin-Vicente C, Fita I, Gomez-Fernandez JC, Verdaguer N, Corbalan-Garcia S. Structural and mechanistic insights into the association of PKC{alpha}-C2 domain to PtdIns(4,5)P2. Proc Natl Acad Sci U S A. 2009 Apr 3. PMID:19346474
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Joel L. Sussman, Jaime Prilusky