Rop protein
From Proteopedia
(Difference between revisions)
| (3 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | + | <StructureSection load='1rop' size='350' side='right' scene='' caption='E. coli Rop protein [[1rop]]'> | |
| + | ==Function== | ||
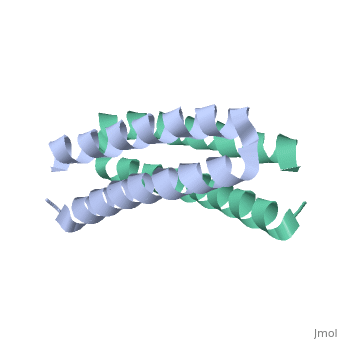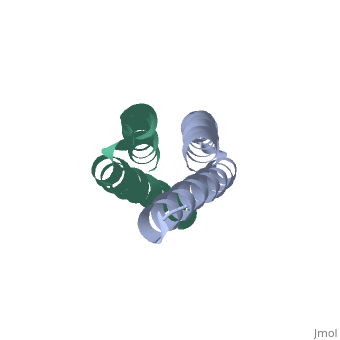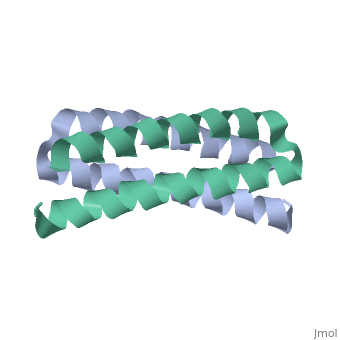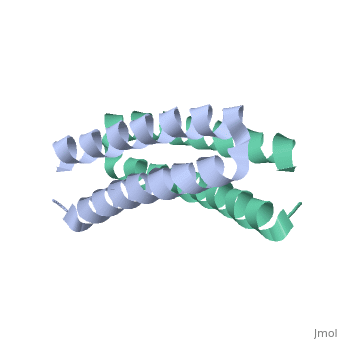
<scene name='Rop_protein/Wt_rop/1'>Rop</scene> '''(Repressor Of Primer)''' is a small homodimeric RNA-binding protein that is involved in the regulation of copy number of the ColE1 plasmids of E.coli, where it is encoded<ref>pmid 2462471</ref>. Its structure has been studied using both X-ray crystallography<ref>PMID:3681971</ref> and NMR<ref>PMID:1841691</ref>. | <scene name='Rop_protein/Wt_rop/1'>Rop</scene> '''(Repressor Of Primer)''' is a small homodimeric RNA-binding protein that is involved in the regulation of copy number of the ColE1 plasmids of E.coli, where it is encoded<ref>pmid 2462471</ref>. Its structure has been studied using both X-ray crystallography<ref>PMID:3681971</ref> and NMR<ref>PMID:1841691</ref>. | ||
Each monomer has molecular weight of about 7.500 Da and it consists of 63 amino acids that form two α-helices connected by <scene name='Rop_protein/Wt_rop_loop/2'> a loop </scene>of four amino acids (L29, D30, A31, D32). The two monomers are related with a 2-fold symmetry axis. | Each monomer has molecular weight of about 7.500 Da and it consists of 63 amino acids that form two α-helices connected by <scene name='Rop_protein/Wt_rop_loop/2'> a loop </scene>of four amino acids (L29, D30, A31, D32). The two monomers are related with a 2-fold symmetry axis. | ||
| Line 16: | Line 17: | ||
*re-engineering topology of the homodimeric ROP protein into a single-chain 4-helix bundle([[1yo7]]) | *re-engineering topology of the homodimeric ROP protein into a single-chain 4-helix bundle([[1yo7]]) | ||
*ALA2ILE2-6, repacted the hydrophobic core and a new fold ([[1f4n]]) | *ALA2ILE2-6, repacted the hydrophobic core and a new fold ([[1f4n]]) | ||
| - | + | </StructureSection> | |
==3D structures of Rop protein== | ==3D structures of Rop protein== | ||
| Line 22: | Line 23: | ||
[[1rpo]], [[1rop]], [[2ghy]] – EcRop – ''Escherichia coli''<br /> | [[1rpo]], [[1rop]], [[2ghy]] – EcRop – ''Escherichia coli''<br /> | ||
| - | [[1qx8]], [[1nkd]], [[1b6q]], [[1yo7]], [[1f4n]], [[3k79]], [[2ijh]], [[2iji]], [[2ijj]], [[2ijk]], [[1gmg]], [[1f4m]], [[4do2]], [[1gto]] – EcRop (mutant)<br /> | + | [[1qx8]], [[1nkd]], [[1b6q]], [[1yo7]], [[1f4n]], [[3k79]], [[2ijh]], [[2iji]], [[2ijj]], [[2ijk]], [[1gmg]], [[1f4m]], [[4do2]], [[1gto]], [[7kae]] – EcRop (mutant)<br /> |
[[1rpr]] – EcRop – NMR<br /> | [[1rpr]] – EcRop – NMR<br /> | ||
[[3q5z]] – TgRop5B pseudokinase domain – ''Toxoplasma gondii'' <br /> | [[3q5z]] – TgRop5B pseudokinase domain – ''Toxoplasma gondii'' <br /> | ||
Current revision
| |||||||||||
3D structures of Rop protein
Updated on 09-March-2022
1rpo, 1rop, 2ghy – EcRop – Escherichia coli
1qx8, 1nkd, 1b6q, 1yo7, 1f4n, 3k79, 2ijh, 2iji, 2ijj, 2ijk, 1gmg, 1f4m, 4do2, 1gto, 7kae – EcRop (mutant)
1rpr – EcRop – NMR
3q5z – TgRop5B pseudokinase domain – Toxoplasma gondii
3q60 – TgRop5B pseudokinase domain + ATP
Additional Resources
For additional information, see: DNA Replication, Repair, and Recombination
References
- ↑ Polisky B. ColE1 replication control circuitry: sense from antisense. Cell. 1988 Dec 23;55(6):929-32. PMID:2462471
- ↑ Banner DW, Kokkinidis M, Tsernoglou D. Structure of the ColE1 rop protein at 1.7 A resolution. J Mol Biol. 1987 Aug 5;196(3):657-75. PMID:3681971
- ↑ Eberle W, Pastore A, Sander C, Rosch P. The structure of ColE1 rop in solution. J Biomol NMR. 1991 May;1(1):71-82. PMID:1841691
Proteopedia Page Contributors and Editors (what is this?)
Maria Amprazi, Michal Harel, Nicole R Pendini, Alexander Berchansky, Keith Callenberg, Jaime Prilusky, David Canner