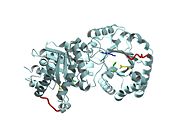
Triosephosphate Isomerase
From Proteopedia
(Difference between revisions)
| (2 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | {{STRUCTURE_1ypi|PDB=1ypi|SCENE=}} | ||
| - | |||
===TRIOSEPHOSPHATE ISOMERASE (TIM, or TPI)=== | ===TRIOSEPHOSPHATE ISOMERASE (TIM, or TPI)=== | ||
| - | + | <StructureSection load='1ypi' size='340' side='right' caption='[[1ypi]], [[Resolution|resolution]] 2.30Å' scene=''> | |
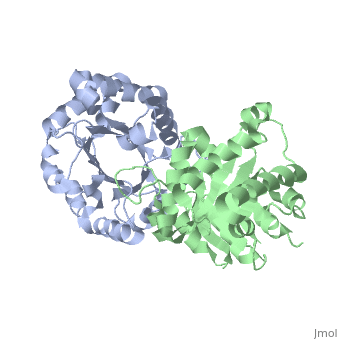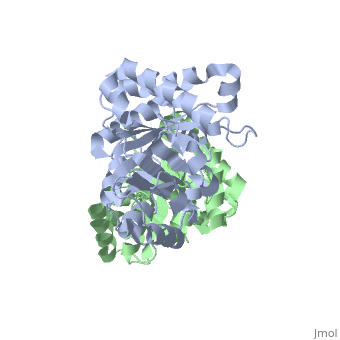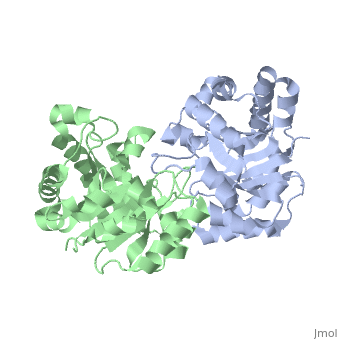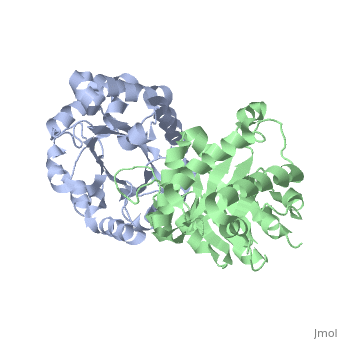
'''Triosephosphate isomerase''' is a key enzyme in the glycolytic pathway, which catalyzes the interconversion of dihydroxyacetone phosphate ([http://en.wikipedia.org/wiki/DHAP DHAP]) and (''R'')-glyceraldehyde-3-phosphate ([http://en.wikipedia.org/wiki/Glycerate_3-phosphate GAP]). The remarkable specificity and catalytic power of the enzyme has inspired intensive studies using structural biology, biophysics, and computer simulations. TIM is a [http://en.wikipedia.org/wiki/Catalytically_perfect_enzyme catalytically perfect enzyme] in the sense that its kcat/Km value is in the diffusion-limited range, and because catalytic efficiency is not improved by changes to the chemical composition of the solvent, or by changes to the amino acid sequence of the enzyme. TIM has been estimated to lower the activation energy of the reaction by 11-13 kcal/mol. | '''Triosephosphate isomerase''' is a key enzyme in the glycolytic pathway, which catalyzes the interconversion of dihydroxyacetone phosphate ([http://en.wikipedia.org/wiki/DHAP DHAP]) and (''R'')-glyceraldehyde-3-phosphate ([http://en.wikipedia.org/wiki/Glycerate_3-phosphate GAP]). The remarkable specificity and catalytic power of the enzyme has inspired intensive studies using structural biology, biophysics, and computer simulations. TIM is a [http://en.wikipedia.org/wiki/Catalytically_perfect_enzyme catalytically perfect enzyme] in the sense that its kcat/Km value is in the diffusion-limited range, and because catalytic efficiency is not improved by changes to the chemical composition of the solvent, or by changes to the amino acid sequence of the enzyme. TIM has been estimated to lower the activation energy of the reaction by 11-13 kcal/mol. | ||
| Line 19: | Line 17: | ||
<html5media height=“854” width=“480”>https://www.youtube.com/embed/XaRpZDsianE</html5media> Movie 2 shows the binding of DHAP and the changes that occur during catalysis. Note the motions in Glu165 (yellow), His95 (green) and Lys12 (blue) on binding of DHAP. This movie was creating by morphing between unbound and bound TIM (PDB ID 1YPI and 2YPI, respectively), and rendered using Molscript and Raster3D. | <html5media height=“854” width=“480”>https://www.youtube.com/embed/XaRpZDsianE</html5media> Movie 2 shows the binding of DHAP and the changes that occur during catalysis. Note the motions in Glu165 (yellow), His95 (green) and Lys12 (blue) on binding of DHAP. This movie was creating by morphing between unbound and bound TIM (PDB ID 1YPI and 2YPI, respectively), and rendered using Molscript and Raster3D. | ||
| - | [[Image:040309a_1.jpg|thumb|[ | + | [[Image:040309a_1.jpg|thumb|This [https://www.youtube.com/embed/XaRpZDsianE movie] shows the binding of DHAP and the changes that occur during catalysis.]] |
Also see hinge motion via Mark Gerstein's Molecular Movements database [ [http://www.molmovdb.org/cgi-bin/morph.cgi?ID=223417-6867] ]. The residues in the flexible loop are highly conserved among a variety of TIM enzymes from different organisms as an indication of their functional significance. Four residues in the flexible loop were deleted by site-directed mutagenesis to test the functional role of the loop region in catalysis. The deletion mutant was found to have a severe defect in kcat despite a modest defect in Km indicating a primary role for the loop region. Furthermore, a significant increase in the level of methylglyoxal was observed for the deletion mutant compared to the wild type enzyme. Methylglyoxal is a toxic by-product of the reaction, which builds up when the enediol intermediate is released from the active site. Thus, the flexible loop also functions to hold the intermediate in the active site so catalysis can proceed to the final product. | Also see hinge motion via Mark Gerstein's Molecular Movements database [ [http://www.molmovdb.org/cgi-bin/morph.cgi?ID=223417-6867] ]. The residues in the flexible loop are highly conserved among a variety of TIM enzymes from different organisms as an indication of their functional significance. Four residues in the flexible loop were deleted by site-directed mutagenesis to test the functional role of the loop region in catalysis. The deletion mutant was found to have a severe defect in kcat despite a modest defect in Km indicating a primary role for the loop region. Furthermore, a significant increase in the level of methylglyoxal was observed for the deletion mutant compared to the wild type enzyme. Methylglyoxal is a toxic by-product of the reaction, which builds up when the enediol intermediate is released from the active site. Thus, the flexible loop also functions to hold the intermediate in the active site so catalysis can proceed to the final product. | ||
| Line 67: | Line 65: | ||
Biochemistry. 1990 Apr 3;29(13):3186-94. | Biochemistry. 1990 Apr 3;29(13):3186-94. | ||
PMID: 2185832 | PMID: 2185832 | ||
| + | |||
| + | |||
| + | |||
| + | </StructureSection> | ||
Current revision
TRIOSEPHOSPHATE ISOMERASE (TIM, or TPI)
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Paula Grabowski, Jacqueline Townsend, Joel L. Sussman, Kara Pryke, Regina D. Kettering, Gregg Snider