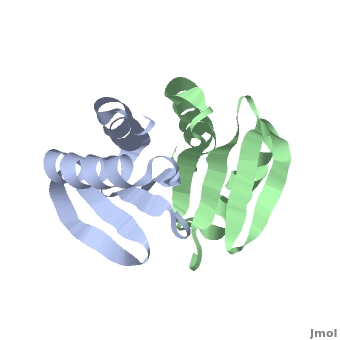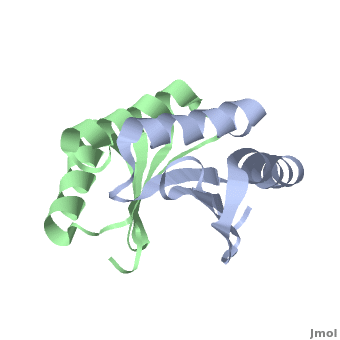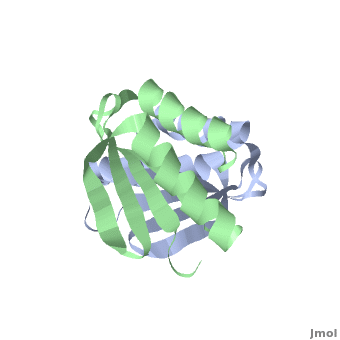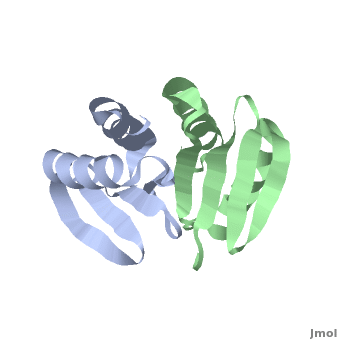We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
TolR
From Proteopedia
(Difference between revisions)
| (5 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | + | <StructureSection load='' size='350' side='right' scene='TolR/Tolrstructure/1' caption='TolR periplasmic domain dimer (PDB code [[2jwk]])'> | |
| - | + | ||
==Structure== | ==Structure== | ||
| - | '''TolR''' is a bitopic protein located in the inner membrane and consists of three domains: domain I (from residues 1 to 43 and including the transmembrane domain between residues 23 to 43), domain II and domain III from residues 117 to 142<ref name='Journet'>PMID: 10419942</ref>. Domains II and III have the ability to dimerize, but unlike domains I and III, domain II is poorly conserved<ref>PMID: 11994151</ref>. For additional details see [[Tol]]. | + | '''TolR''' is a bitopic protein (protein having a single α helix in its transmembrane domain) located in the inner membrane and consists of three domains: domain I (from residues 1 to 43 and including the transmembrane domain between residues 23 to 43), domain II and domain III from residues 117 to 142<ref name='Journet'>PMID: 10419942</ref>. Domains II and III have the ability to dimerize, but unlike domains I and III, domain II is poorly conserved<ref>PMID: 11994151</ref>. For additional details see [[Tol]]. |
Domain III has been suggested to form an amphiphilic α-helix, which play an essential role in the functional assembly of the [[Tol]] complex<ref name='Lazzaroni'>PMID: 7853390</ref>. | Domain III has been suggested to form an amphiphilic α-helix, which play an essential role in the functional assembly of the [[Tol]] complex<ref name='Lazzaroni'>PMID: 7853390</ref>. | ||
| Line 13: | Line 12: | ||
# [[Colicin A]] import | # [[Colicin A]] import | ||
# Maintaining cell envelope integrity | # Maintaining cell envelope integrity | ||
| + | </StructureSection> | ||
| + | ==3D structures of TolR== | ||
| - | + | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | |
[[2jwk]], [[2jwl]] – TolR periplasmic domain – ''Haemophilus influenzae'' – NMR<br /> | [[2jwk]], [[2jwl]] – TolR periplasmic domain – ''Haemophilus influenzae'' – NMR<br /> | ||
| - | [[5by4]] – | + | [[5by4]] – EcTolR – ''Escherichia coli''<br /> |
| + | [[8odt]] – EcTolR + TolQ – Cryo EM<br /> | ||
==References== | ==References== | ||
<references/> | <references/> | ||
| + | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
3D structures of TolR
Updated on 27-December-2023
2jwk, 2jwl – TolR periplasmic domain – Haemophilus influenzae – NMR
5by4 – EcTolR – Escherichia coli
8odt – EcTolR + TolQ – Cryo EM
References
- ↑ 1.0 1.1 1.2 1.3 Journet L, Rigal A, Lazdunski C, Benedetti H. Role of TolR N-terminal, central, and C-terminal domains in dimerization and interaction with TolA and tolQ. J Bacteriol. 1999 Aug;181(15):4476-84. PMID:10419942
- ↑ Walburger A, Lazdunski C, Corda Y. The Tol/Pal system function requires an interaction between the C-terminal domain of TolA and the N-terminal domain of TolB. Mol Microbiol. 2002 May;44(3):695-708. PMID:11994151
- ↑ 3.0 3.1 Lazzaroni JC, Vianney A, Popot JL, Benedetti H, Samatey F, Lazdunski C, Portalier R, Geli V. Transmembrane alpha-helix interactions are required for the functional assembly of the Escherichia coli Tol complex. J Mol Biol. 1995 Feb 10;246(1):1-7. PMID:7853390 doi:http://dx.doi.org/10.1006/jmbi.1994.0058