Victrelis (boceprevir)
From Proteopedia
| (39 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
==Hepatitis C Virus== | ==Hepatitis C Virus== | ||
The Hepatitis C virus (HCV) is a blood-borne, single stranded RNA virus. <ref>Centers for Disease Control and Prevention. (2015). Viral hepatitis - hepatitis C information. Retrieved from http://www.cdc.gov/hepatitis/hcv/index.htm</ref> Chronic Hepatitis C infection is a leading cause of mortality and morbidity across the globe, affecting between 130-170 million people. <ref>doi: 10.1093/cid/cis361</ref> The genome of HCV encodes a polyprotein precursor of approximately 3,000 amino acids, which are cleaved into 10 proteins by various proteases, one of which is the non-structural protein 3 (NS3) serine protease.<ref name="Line">PMID:21250386</ref> | The Hepatitis C virus (HCV) is a blood-borne, single stranded RNA virus. <ref>Centers for Disease Control and Prevention. (2015). Viral hepatitis - hepatitis C information. Retrieved from http://www.cdc.gov/hepatitis/hcv/index.htm</ref> Chronic Hepatitis C infection is a leading cause of mortality and morbidity across the globe, affecting between 130-170 million people. <ref>doi: 10.1093/cid/cis361</ref> The genome of HCV encodes a polyprotein precursor of approximately 3,000 amino acids, which are cleaved into 10 proteins by various proteases, one of which is the non-structural protein 3 (NS3) serine protease.<ref name="Line">PMID:21250386</ref> | ||
| - | |||
| - | <Structure load='2oc8' size='400' frame='true' align='right' caption='Structure of Hepatitis C Viral NS3 protease domain complexed with NS4A peptide and ketoamide SCH503034 (PDB code [[2oc8]])' scene='Insert optional scene name here' /> | ||
==Victrelis== | ==Victrelis== | ||
| - | Victrelis (boceprevir) is an antiviral Hepatitis C medication approved by the United States Food and Drug Administration in 2011. Victrelis is an inhibitor of the NS3 serine protease.<ref name="Merck">Merck & Co., Inc. (n.d.) Highlights of prescribing information. Retrieved from https://www.merck.com/product/usa/pi_circulars/v/victrelis/victrelis_pi.pdf</ref> | + | Victrelis (boceprevir) is an antiviral Hepatitis C medication manufactured by Merck & Co., Inc. and approved by the United States Food and Drug Administration in 2011. Victrelis is an inhibitor of the NS3 serine protease. Inhibition of this protease prevents viral replication in Hepatitis C virus infected cells. <ref name="Merck">Merck & Co., Inc. (n.d.) Highlights of prescribing information. Retrieved from https://www.merck.com/product/usa/pi_circulars/v/victrelis/victrelis_pi.pdf</ref> For greatest effectiveness, Victrelis is prescribed along with two other drugs, ribavirin and pegylated interferon, to improve the effectiveness of Victrelis and activate the body's immune response, respectively.<ref name="Info">InfoHep. (2016). Hepatitis C treatment factsheet: Boceprevir (Victrelis). Retrieved from http://www.infohep.org/Hepatitis-C-treatment-factsheet-Boceprevir-iVictrelisi/page/2845312/</ref> When used in combination with ribavirin and pegylated interferon in clinical trials, two-thirds of patients did not have detectable HCV in their blood after 24 weeks of treatment.<ref>Food and Drug Administration. (2011). FDA approves Victrelis for Hepatitis C. Retrieved from http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm255390.htm</ref> In 2015, Merck & Co., Inc. announced that they would no longer sell the drug in the United States as of January 2016 due to a new, more lucrative family of drugs develped for HCV treatment.<ref name="NYT">Loftus, Peter. (2015). Merck will no longer sell its Hepatitis C drug in the US. Retrieved from http://blogs.wsj.com/pharmalot/2015/01/21/merck-will-no-longer-sell-its-victrelis-hepatitis-c-drug-in-the-u-s/</ref> However, Victrelis is still widely prescribed, in combination with ribavirin and pegylated interferon, in other countries, including the United Kingdom.<ref name="NYT" /><ref name="Info" /> |
| - | == Structure of Victrelis == | + | <Structure load='2oc8' size='500' frame='true' align='right' caption='Structure of Hepatitis C Viral NS3 protease domain complexed with NS4A peptide and Victrelis (PDB code [[2oc8]])' scene='Insert optional scene name here' /> |
| + | |||
| + | ==Function of Serine Proteases== | ||
| + | Serine proteases catalyze the cleavage of peptide bonds and are found in both eukaryotes and prokaryotes.<ref name="Brandt">Brandt, Mark. (2016). Enzyme mechanisms. Retrieved from https://www.rose-hulman.edu/~brandt/Chem330/Enzyme_mech_examples.pdf</ref><ref name="SERINE">doi:10.1002/iub.186</ref> Typically, serine proteases catalyze hydrolysis of peptide bonds in the middle of polypeptide chains.<ref name="SERINE" /> All serine proteases have a nucleophilic serine in the active site.<ref name="SERINE" /> The active site serine becomes nucleophilic through interactions with a catalytic triad that is composed of aspartic acid, histidine, and serine.<ref name="SERINE" /> Once nuclophilic, the serine attacks carbonyl components of substrate peptide bonds, creating an acyl-enzyme intermediate.<ref name="ACS">doi:10.1021/cr000033x</ref> An oxyanion hole, formed by interactions between the active site serine and a glycine residue, forms a small space of positive charge that stabilizes negatively charged intermediates.<ref name="ACS" /> | ||
| + | |||
| + | ==Structure of Unbound Victrelis == | ||
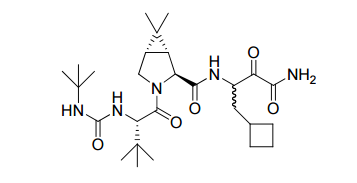
The active ingredient in Victrelis, boceprevir, is an equal mixture of two diastereomers made of entirely organic molecules. The molecular formula is C27H45N5O5, and the molecular weight is 519.8 grams/mole. The structural formula is as follows: <ref name="Merck" /> | The active ingredient in Victrelis, boceprevir, is an equal mixture of two diastereomers made of entirely organic molecules. The molecular formula is C27H45N5O5, and the molecular weight is 519.8 grams/mole. The structural formula is as follows: <ref name="Merck" /> | ||
[[Image:Victrelis.png]] | [[Image:Victrelis.png]] | ||
| - | + | == Structure of Bound Victrelis & Mechanism of Action == | |
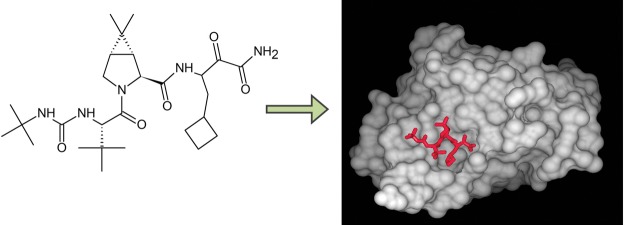
| - | + | Victrelis is a protease inhibitor.<ref name="Merck" /> A protease is an enzyme that cleaves large polypeptides to create fragments that are small enough to be sequenced for effective replication, and are essential to viral RNA replication.<ref name="Voet">Voet, D., Voet, J., Pratt, C. (2013). Fundamentals of biochemistry: life at the molecular level (4th ed.). Hoboken, New Jersey: John Wiley & Sons, Inc.</ref> Victrelis specifically inhibits the HCV NS3/4A protease, which is responsible for cleavage at four different sites along the polyprotein: NS3/NS4A, NS4A/NS4B, NS4B/NS5A, and NS5A/NS5B.<ref name="Line" /> By inhibiting cleavage at these sites, smaller protein fragments are unable to be produced, causing a halt in RNA replication due to the large size of the polyproteins. The active ingredient in Victrelis, boceprevir, covalently binds to the active site serine <scene name='74/746128/Covalently_bound/3'>(Ser139)</scene> in the NS3 protease.<ref name="Line" /> The nucleophilic oxygen in the hydroxyl of the Ser139 group binds to Victrelis at the carbon of the carbonyl group located two carbons away from the terminal amine.<ref name="Line" /> | |
| - | + | The Ser139 residue of the NS3 serine protease participates in the formation of two critical structures for serine protease function: the catalytic triad and the oxyanion hole.<ref name="Line" /> The <scene name='74/746128/Catalytic_triad/2'>catalytic triad</scene> is formed through interactions with Ser139, His57, and Asp81.<ref name="Line" /> In order to form the triad, the carboxylic group of Asp81 forms a hydrogen bond with His57, increasing the pKa of the histidine side chain from 7 to 12. With a pKa of 12, His57 then deprotonates the hydroxyl group of Ser139, allowing the deprotonated serine side chain to function as a nucleophile.<ref name="Brandt" /ref> The nucleophilic Ser139 is now able to attack carbonyl carbons of various substrates, including Victrelis, while the protonated His57 is able to cleave substrates and collapse intermediates.<ref name="Brandt" /> The oxyanion hole is formed when Ser139 hydrogen bonds to Gly137, and is crucial to stabilize negative intermediates.<ref name="Line" /> | |
| - | + | ||
| - | In previous experiments, when any member of the catalytic | + | Victrelis covalently, yet reversibly, binds to the NS3 protease at the <scene name='74/746128/Covalently_bound/3'>Ser139</scene> residue, thereby prohibiting interactions between the oxyanion hole and negative intermediates, as well as the catalytic triad and substrates.<ref name="Line" /> When bound to the NS3 serine protease, Victrelis also participates in <scene name='74/746128/Hydrophobic_interactions/1'>hydrophobic interactions</scene> between the side chains of specific amino acid residues on the NS3 serine protease: Gln41, Gly137, Arg155, Ala156, and Asp168.<ref name="Line" /> The reactive carbonyl group at the beta-carbon position, located two carbons away from the terminal amine group, of Victrelis is attacked by the nucleophilic oxygen in Ser139 to create a <scene name='74/746128/Covalently_bound/3'>covalent</scene> bond.<ref>doi:10.2210/pdb2oc8/pdb</ref> |
| + | |||
| + | In previous experiments, when any member of the catalytic triad was replaced by a different residue, cleavage at the NS3/NS4A, NS4A/NS4B, NS4B/NS5A, and NS5A/NS5B locations was inhibited.<ref name="Line" /> By inhibiting the protease from cleaving the HCV at the NS3/NS4A location, the polyprotein is unable to be cleaved into smaller, functional proteins that compose replications machinery of HCV, thereby inhibiting viral replication.<ref name="Howe">doi:10.14218/JCTH.2013.002XX</ref> In addition to viruses, mammals also utilize serine proteases to cleave lengthy polypeptides that are involved in various essential mechanisms throughout the body.<ref name="Voet" /> Victrelis was testing among several different mammalian proteases, including those that are vital for blood clotting, digestion, and antibody neutralization, and it was found to be exclusively selective for inhibition of the NS3 serine protease of HCV only.<ref name="Howe" /> | ||
| + | |||
| + | [[Image:Boceprevir bound to NS3 active site.jpg]] | ||
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
Contents |
Hepatitis C Virus
The Hepatitis C virus (HCV) is a blood-borne, single stranded RNA virus. [1] Chronic Hepatitis C infection is a leading cause of mortality and morbidity across the globe, affecting between 130-170 million people. [2] The genome of HCV encodes a polyprotein precursor of approximately 3,000 amino acids, which are cleaved into 10 proteins by various proteases, one of which is the non-structural protein 3 (NS3) serine protease.[3]
Victrelis
Victrelis (boceprevir) is an antiviral Hepatitis C medication manufactured by Merck & Co., Inc. and approved by the United States Food and Drug Administration in 2011. Victrelis is an inhibitor of the NS3 serine protease. Inhibition of this protease prevents viral replication in Hepatitis C virus infected cells. [4] For greatest effectiveness, Victrelis is prescribed along with two other drugs, ribavirin and pegylated interferon, to improve the effectiveness of Victrelis and activate the body's immune response, respectively.[5] When used in combination with ribavirin and pegylated interferon in clinical trials, two-thirds of patients did not have detectable HCV in their blood after 24 weeks of treatment.[6] In 2015, Merck & Co., Inc. announced that they would no longer sell the drug in the United States as of January 2016 due to a new, more lucrative family of drugs develped for HCV treatment.[7] However, Victrelis is still widely prescribed, in combination with ribavirin and pegylated interferon, in other countries, including the United Kingdom.[7][5]
|
Function of Serine Proteases
Serine proteases catalyze the cleavage of peptide bonds and are found in both eukaryotes and prokaryotes.[8][9] Typically, serine proteases catalyze hydrolysis of peptide bonds in the middle of polypeptide chains.[9] All serine proteases have a nucleophilic serine in the active site.[9] The active site serine becomes nucleophilic through interactions with a catalytic triad that is composed of aspartic acid, histidine, and serine.[9] Once nuclophilic, the serine attacks carbonyl components of substrate peptide bonds, creating an acyl-enzyme intermediate.[10] An oxyanion hole, formed by interactions between the active site serine and a glycine residue, forms a small space of positive charge that stabilizes negatively charged intermediates.[10]
Structure of Unbound Victrelis
The active ingredient in Victrelis, boceprevir, is an equal mixture of two diastereomers made of entirely organic molecules. The molecular formula is C27H45N5O5, and the molecular weight is 519.8 grams/mole. The structural formula is as follows: [4]
Structure of Bound Victrelis & Mechanism of Action
Victrelis is a protease inhibitor.[4] A protease is an enzyme that cleaves large polypeptides to create fragments that are small enough to be sequenced for effective replication, and are essential to viral RNA replication.[11] Victrelis specifically inhibits the HCV NS3/4A protease, which is responsible for cleavage at four different sites along the polyprotein: NS3/NS4A, NS4A/NS4B, NS4B/NS5A, and NS5A/NS5B.[3] By inhibiting cleavage at these sites, smaller protein fragments are unable to be produced, causing a halt in RNA replication due to the large size of the polyproteins. The active ingredient in Victrelis, boceprevir, covalently binds to the active site serine in the NS3 protease.[3] The nucleophilic oxygen in the hydroxyl of the Ser139 group binds to Victrelis at the carbon of the carbonyl group located two carbons away from the terminal amine.[3]
The Ser139 residue of the NS3 serine protease participates in the formation of two critical structures for serine protease function: the catalytic triad and the oxyanion hole.[3] The is formed through interactions with Ser139, His57, and Asp81.[3] In order to form the triad, the carboxylic group of Asp81 forms a hydrogen bond with His57, increasing the pKa of the histidine side chain from 7 to 12. With a pKa of 12, His57 then deprotonates the hydroxyl group of Ser139, allowing the deprotonated serine side chain to function as a nucleophile.[8]
In previous experiments, when any member of the catalytic triad was replaced by a different residue, cleavage at the NS3/NS4A, NS4A/NS4B, NS4B/NS5A, and NS5A/NS5B locations was inhibited.[3] By inhibiting the protease from cleaving the HCV at the NS3/NS4A location, the polyprotein is unable to be cleaved into smaller, functional proteins that compose replications machinery of HCV, thereby inhibiting viral replication.[12] In addition to viruses, mammals also utilize serine proteases to cleave lengthy polypeptides that are involved in various essential mechanisms throughout the body.[11] Victrelis was testing among several different mammalian proteases, including those that are vital for blood clotting, digestion, and antibody neutralization, and it was found to be exclusively selective for inhibition of the NS3 serine protease of HCV only.[12]
References
- ↑ Centers for Disease Control and Prevention. (2015). Viral hepatitis - hepatitis C information. Retrieved from http://www.cdc.gov/hepatitis/hcv/index.htm
- ↑ Averhoff FM, Glass N, Holtzman D. Global burden of hepatitis C: considerations for healthcare providers in the United States. Clin Infect Dis. 2012 Jul;55 Suppl 1:S10-5. doi: 10.1093/cid/cis361. PMID:22715208 doi:http://dx.doi.org/10.1093/cid/cis361
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Lin C. HCV NS3-4A Serine Protease PMID:21250386
- ↑ 4.0 4.1 4.2 Merck & Co., Inc. (n.d.) Highlights of prescribing information. Retrieved from https://www.merck.com/product/usa/pi_circulars/v/victrelis/victrelis_pi.pdf
- ↑ 5.0 5.1 InfoHep. (2016). Hepatitis C treatment factsheet: Boceprevir (Victrelis). Retrieved from http://www.infohep.org/Hepatitis-C-treatment-factsheet-Boceprevir-iVictrelisi/page/2845312/
- ↑ Food and Drug Administration. (2011). FDA approves Victrelis for Hepatitis C. Retrieved from http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm255390.htm
- ↑ 7.0 7.1 Loftus, Peter. (2015). Merck will no longer sell its Hepatitis C drug in the US. Retrieved from http://blogs.wsj.com/pharmalot/2015/01/21/merck-will-no-longer-sell-its-victrelis-hepatitis-c-drug-in-the-u-s/
- ↑ 8.0 8.1 Brandt, Mark. (2016). Enzyme mechanisms. Retrieved from https://www.rose-hulman.edu/~brandt/Chem330/Enzyme_mech_examples.pdf
- ↑ 9.0 9.1 9.2 9.3 Di Cera E. Serine proteases. IUBMB Life. 2009 May;61(5):510-5. PMID:19180666 doi:10.1002/iub.186
- ↑ 10.0 10.1 doi: https://dx.doi.org/10.1021/cr000033x
- ↑ 11.0 11.1 Voet, D., Voet, J., Pratt, C. (2013). Fundamentals of biochemistry: life at the molecular level (4th ed.). Hoboken, New Jersey: John Wiley & Sons, Inc.
- ↑ 12.0 12.1 Howe AY, Venkatraman S. The Discovery and Development of Boceprevir: A Novel, First-generation Inhibitor of the Hepatitis C Virus NS3/4A Serine Protease. J Clin Transl Hepatol. 2013 Sep;1(1):22-32. doi: 10.14218/JCTH.2013.002XX. Epub, 2013 Sep 15. PMID:26357603 doi:http://dx.doi.org/10.14218/JCTH.2013.002XX