Fosamax (alendronate sodium)
From Proteopedia
| (22 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | <scene name='74/745950/Correct_ligand_binding_site/3'></scene><table style="width:200px; border:1px solid black; float:right; "> | + | <scene name='74/745950/Correct_ligand_binding_site/8'></scene><scene name='74/745950/Correct_ligand_binding_site/3'></scene><table style="width:200px; border:1px solid black; float:right; "> |
<tr> | <tr> | ||
<td colspan="2"><center> FOSAMAX </center></td> | <td colspan="2"><center> FOSAMAX </center></td> | ||
| Line 30: | Line 30: | ||
Fosamax (Alendronate Sodium or Alendronic Acid) is a pharmaceutical drug administered as an antiresorptive therapy agent for osteodegenerative diseases such as [https://en.wikipedia.org/wiki/Osteoporosis Osteoporosis], [https://en.wikipedia.org/wiki/Paget's_disease_of_bone Paget's disease], and [https://en.wikipedia.org/wiki/Osteogenesis_imperfecta osteogenesis imperfecta]<ref>Gong Li, Altman Russ B, Klein Teri E . "Bisphosphonates pathway" Pharmacogenetics and genomics (2011). from https://www.pharmgkb.org/pathway/PA154423660</ref>. | Fosamax (Alendronate Sodium or Alendronic Acid) is a pharmaceutical drug administered as an antiresorptive therapy agent for osteodegenerative diseases such as [https://en.wikipedia.org/wiki/Osteoporosis Osteoporosis], [https://en.wikipedia.org/wiki/Paget's_disease_of_bone Paget's disease], and [https://en.wikipedia.org/wiki/Osteogenesis_imperfecta osteogenesis imperfecta]<ref>Gong Li, Altman Russ B, Klein Teri E . "Bisphosphonates pathway" Pharmacogenetics and genomics (2011). from https://www.pharmgkb.org/pathway/PA154423660</ref>. | ||
| - | Functioning as a nitrogen-containing, second generation [https://en.wikipedia.org/wiki/Bisphosphonate bisphosphonate], Fosamax binds to [https://en.wikipedia.org/wiki/Hydroxylapatite hydroxyapatite] in bone and promotes [https://en.wikipedia.org/wiki/Apoptosis apoptosis] in [https://en.wikipedia.org/wiki/Osteoclast osteoclasts] (cells specialized in skeletal breakdown), thereby delaying the degradation of | + | Functioning as a nitrogen-containing, second generation [https://en.wikipedia.org/wiki/Bisphosphonate bisphosphonate], Fosamax binds to [https://en.wikipedia.org/wiki/Hydroxylapatite hydroxyapatite] in bone and promotes [https://en.wikipedia.org/wiki/Apoptosis apoptosis] in [https://en.wikipedia.org/wiki/Osteoclast osteoclasts] (cells specialized in skeletal breakdown), thereby delaying the degradation of osseous tissue. While incorporated in the bone matrix, alendronate is not pharmacologically active; therefore, it must be continuously administered to suppress osteoclasts on newly formed resorption surfaces <ref>Fosamax: Uses, Dosage & Side Effects - Drugs.com. (2016, November 3).Fosamax: Uses, Dosage & Side Effects - Drugs.com. Retrieved November 14, 2016, from https://www.drugs.com/fosamax.html</ref>. |
== History <ref>Fosamax (alendronate sodium). Fosamax New FDA Drug Approval | CenterWatch. Merck. Retrieved November 15, 2016, from http://www.centerwatch.com/drug-information/fda-approved-drugs/drug/26/fosamax-alendronate-sodium</ref>== | == History <ref>Fosamax (alendronate sodium). Fosamax New FDA Drug Approval | CenterWatch. Merck. Retrieved November 15, 2016, from http://www.centerwatch.com/drug-information/fda-approved-drugs/drug/26/fosamax-alendronate-sodium</ref>== | ||
| Line 37: | Line 37: | ||
== Side Effects <ref>Common Side Effects of Fosamax (Alendronate Sodium) Drug Center - RxList. (2015, August 26). RxList. Retrieved November 14, 2016, from http://www.rxlist.com/fosamax-side-effects-drug-center.htm</ref> == | == Side Effects <ref>Common Side Effects of Fosamax (Alendronate Sodium) Drug Center - RxList. (2015, August 26). RxList. Retrieved November 14, 2016, from http://www.rxlist.com/fosamax-side-effects-drug-center.htm</ref> == | ||
Common side effects of Fosamax include gas, constipation, heartburn, diarrhea, bloating, nausea, vomiting, stomach pain, joint pain or swelling, swelling in your hands or feet, dizziness, headache, eye pain, back pain, or weakness. | Common side effects of Fosamax include gas, constipation, heartburn, diarrhea, bloating, nausea, vomiting, stomach pain, joint pain or swelling, swelling in your hands or feet, dizziness, headache, eye pain, back pain, or weakness. | ||
| - | Serious side effects of Fosamax include severe pain (joints, bone, muscle, jaw, back or heartburn) | + | Serious [but rare] side effects of Fosamax include severe pain (joints, bone, muscle, jaw, back, or heartburn), difficulty swallowing, bloody stools, eye pain, skin blisters, and swelling of the face, tongue, or throat. |
== Structural Highlights <ref>Protein Data Bank in Europe: Bringing Structure to Biology, from http://www.ebi.ac.uk/pdbe/entry/pdb/2F89</ref> == | == Structural Highlights <ref>Protein Data Bank in Europe: Bringing Structure to Biology, from http://www.ebi.ac.uk/pdbe/entry/pdb/2F89</ref> == | ||
| - | As a bisphosphonate, Fosamax is a synthetic analog of [https://en.wikipedia.org/wiki/Pyrophosphate pyrophosphate], containing two | + | As a bisphosphonate, Fosamax is a synthetic analog of [https://en.wikipedia.org/wiki/Pyrophosphate pyrophosphate], containing two phosphate groups on the central carbon. In its drug form, Fosamax includes a sodium bounded in place of a carboxyl hydrogen. In solution, oxyanions may form an ionic bond(s) with metal cations, such as magnesium. The central carbon is also attached to a hydroxyl group (-OH) and a three-carbon chain [-(CH2)3] leading to an amino group (-NH2) (Fig. 2). |
<StructureSection load='2f89' size='340' side='right' caption='Crystal Structure of human FPPS in complex with a bisphosphonate.' scene=''> [[Image:Screen Shot 2016-12-04 at 3.21.51 PM.png|thumb|left|200px|Figure 2. Chemical structure of Fosamax (alendronate sodium).]] | <StructureSection load='2f89' size='340' side='right' caption='Crystal Structure of human FPPS in complex with a bisphosphonate.' scene=''> [[Image:Screen Shot 2016-12-04 at 3.21.51 PM.png|thumb|left|200px|Figure 2. Chemical structure of Fosamax (alendronate sodium).]] | ||
| - | [[Image:Screen Shot 2016-12-02 at 3.24.13 PM.png|thumb|left|400px|Figure 3. GPP/ | + | [[Image:Screen Shot 2016-12-02 at 3.24.13 PM.png|thumb|left|400px|Figure 3. DMAPP/GPP substrate/product site of SPSS-alendronate complex indicated by yellow eclipse.]] |
[[Image:Screen Shot 2016-12-02 at 3.33.04 PM.png|thumb|left|400px|Figure 4. Aspartate-Magnesium cation-phosphonate chelate within FPPS-alendronate complex. Pink sphere indicates Mg2+ ion; red indicates oxygen atom, yellow indicates phosphorous atom, blue (bond-line) indicates nitrogen, blue (ribbon) indicates alpha helix motif.]] [[Image:Screen Shot 2016-12-04 at 8.42.20 PM.png|thumb|left|500px|Figure 5. Comparison between the structure of the carbocation transition states of DMAPP and GPP to the nitrogen-containing bisphosphonate alendronate. Close similarity suggests that Fosamax acts as carbocation transition state analog of isoprenoid diphosphates <ref>Martin, M. B., Arnold, W., Heath, H. T., III, Urbina, J. A., and Oldfield, E. (1999) Biochem. Biophys. Res. Commun. 263, 754–758</ref>.]] | [[Image:Screen Shot 2016-12-02 at 3.33.04 PM.png|thumb|left|400px|Figure 4. Aspartate-Magnesium cation-phosphonate chelate within FPPS-alendronate complex. Pink sphere indicates Mg2+ ion; red indicates oxygen atom, yellow indicates phosphorous atom, blue (bond-line) indicates nitrogen, blue (ribbon) indicates alpha helix motif.]] [[Image:Screen Shot 2016-12-04 at 8.42.20 PM.png|thumb|left|500px|Figure 5. Comparison between the structure of the carbocation transition states of DMAPP and GPP to the nitrogen-containing bisphosphonate alendronate. Close similarity suggests that Fosamax acts as carbocation transition state analog of isoprenoid diphosphates <ref>Martin, M. B., Arnold, W., Heath, H. T., III, Urbina, J. A., and Oldfield, E. (1999) Biochem. Biophys. Res. Commun. 263, 754–758</ref>.]] | ||
| Line 48: | Line 48: | ||
== Mechanism of Action == | == Mechanism of Action == | ||
| - | Functioning as a nitrogen-containing, second generation bisphosphonate, Fosamax binds to hydroxyapatite in bone and promotes apoptosis in osteoclasts (cells specialized in skeletal breakdown) (Fig. 6), thus slowing the breakdown of | + | Functioning as a nitrogen-containing, second generation bisphosphonate, Fosamax binds to hydroxyapatite in bone and promotes apoptosis in osteoclasts (cells specialized in skeletal breakdown) (Fig. 6), thus slowing the breakdown of osseous tissue<ref>Drake, Matthew T., Bart L. Clarke, and Sundeep Khosla. "Bisphosphonates: Mechanism of Action and Role in Clinical Practice." Mayo Clinic Proceedings 83, no. 9 (2008): 1032-045. doi:10.4065/83.9.1032.</ref>. In order to accomplish this, Fosamax inhibits [http://www.uniprot.org/uniprot/P14324 farnesyl pyrophosphate synthase] (FPPS), an enzyme which plays a role within the [https://en.wikipedia.org/wiki/Mevalonate_pathway mevalonic acid pathway], thereby reducing the production rate of isoprenoid compounds that are essential for post-translational modification of small guanosine triphosphate (GTP)-binding proteins (e.g. Rho, Rab and [https://pdb101.rcsb.org/motm/148 Ras])<ref>Luckman SP, Hughes DE, Coxon FP, Russell RGG, Rogers MJ (2005). Nitrogen-Containing Biphosphonates Inhibit the Mevalonate Pathway and Prevent Post-Translational Prenylation of GTP-Binding Proteins, Including Ras. Journal of Bone and Mineral Research. 20, 1265–74</ref>. By inhibiting FPPS, Fosamax discourages the successive condensation of isopentenyl pyrophosphate with dimethylallyl pyrophosphate to geranyl pyrophosphate, which interferes with osteoclast function and survival <ref>Alendronic acid. (2005, June 13), from http://www.drugbank.ca/drugs/DB00630#bond-15126</ref><ref>Fisher, J. E., Rogers, M. J., Halasy, J. M., Luckman, S. P., Hughes, D. E., Masarachia, P. J., … Reszka, A. A. (1999). Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proceedings of the National Academy of Sciences, 96(1), 133–138. doi:10.1073/pnas.96.1.133, from http://www.pnas.org/content/96/1/133.full.pdf</ref>. [[Image:Screen Shot 2016-12-04 at 8.23.53 PM.png|thumb|right|400px|Figure 6. Relationship between bisphosphonates and osteoclasts at individual stages of cellular life.<ref>Martin, M. B., Arnold, W., Heath, H. T., III, Urbina, J. A., and Oldfield, E. (1999) Biochem. Biophys. Res. Commun. 263, 754–758</ref>]] |
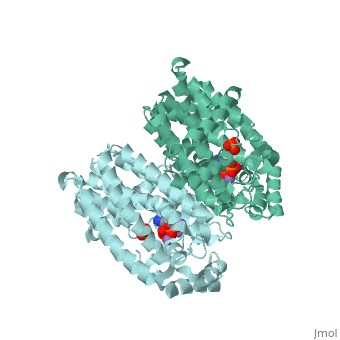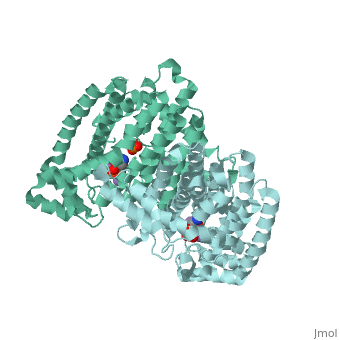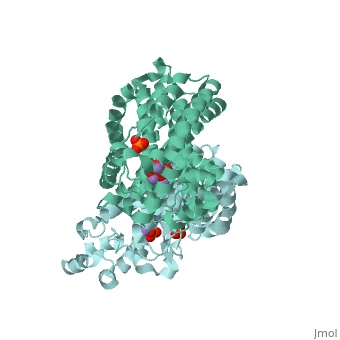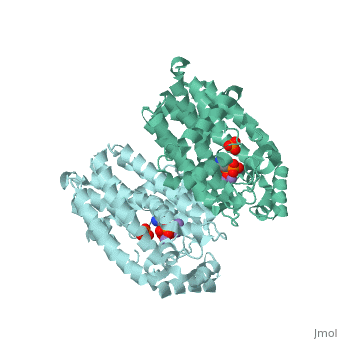
| - | Though the exact chemical mechanism by which alendronate inhibits FPPS remains unclear, nitrogen-containing bisphosphates (<scene name='74/745950/Ligand/ | + | Though the exact chemical mechanism by which alendronate inhibits FPPS remains unclear, nitrogen-containing bisphosphates (<scene name='74/745950/Ligand/4'>N-BP</scene>s) bind to the dimethylallyl/geranyl pyrophosphate (DMAPP/GPP) ligand pocket (Fig. 3) and alter the secondary structure of the enzyme<ref>Kavanagh KL, Guo K, Dunford JE, Wu X, Knapp S, and Ebetino FH (2006). The molecular mechanism of nitrogen-containing bisphosphonates as antiosteoporosis drugs. Proceedings of the National Academy of Sciences. 103, 7829–34</ref>. Specifically, alendronate may bind to the FPPS allylic substrate-binding pocket by ligating the Magnesium ion center via phosphonate oxyanion nucleophilic attack<ref>Hosfield DJ, Zhang Y, Dougan DR, Broun A, Tari LW, Swanson RV (2003). Structural Basis for Bisphosphonate-mediated Inhibition of Isoprenoid Biosynthesis. Journal of Biological Chemistry. 2279, 8526–9</ref>. Phosphonate groups are stabilized by a cluster of three Mg2+ ions via oxyanion-metal cation charge interactions, as well as three FPPS-conserved aspartate residues located within two alpha helical motifs (<scene name='74/745950/Aspartates/4'>Asp-103, 107, and 243</scene>) that form a <scene name='74/745950/Correct_ligand_binding_site/8'>magnesium-bisphosphonate chelate</scene> (Fig. 4)<ref>Tarshis L. C., Yan M., Poulter C. D., Sacchettini J. C. (1994) Biochemistry. 33, 10871–10877.</ref>. N-BPs may bind to the GPP substrate-binding site because N-BPs resemble the structure of the enzyme’s natural substrates (GPP and DMAPP) and act as carbocation transition state analogs (Fig. 5). Interactions between N-BP and amino acid residues (<scene name='74/745950/Lys200_thr201/5'>Lys-200 and Thr-201</scene>) suggest that these inhibitors <scene name='74/745950/Ligand_and_yellow/1'>position their nitrogen group</scene> in the carbocation-binding site, then mimic the carbocation intermediate formed via substrate ionization <ref>Martin, M. B., Arnold, W., Heath, H. T., III, Urbina, J. A., and Oldfield, E. (1999) Biochem. Biophys. Res. Commun. 263, 754–758</ref>. By acting as a transition state analog for isoprenoid biosynthesis, Fosamax effectively increases the activation energy of a regulatory/rate-determining step in the mevalonate pathway, subsequently reducing the concentration of product molecules that aid the continuous biodegradation of skeletal tissue. |
Current revision
| Formula: | C4H13NO7P2 |
| Molar Mass: | 249.097 g/mol |
| INN: | Alendronic Acid |
| USAN: | Alendronate sodium |
| IUPAC: | 4-amino-1-hydroxybutylidene) bisphosphonic acid monosodium salt trihydrate |
Fosamax (Alendronate Sodium or Alendronic Acid) is a pharmaceutical drug administered as an antiresorptive therapy agent for osteodegenerative diseases such as Osteoporosis, Paget's disease, and osteogenesis imperfecta[1]. Functioning as a nitrogen-containing, second generation bisphosphonate, Fosamax binds to hydroxyapatite in bone and promotes apoptosis in osteoclasts (cells specialized in skeletal breakdown), thereby delaying the degradation of osseous tissue. While incorporated in the bone matrix, alendronate is not pharmacologically active; therefore, it must be continuously administered to suppress osteoclasts on newly formed resorption surfaces [2].
Contents |
History [3]
Fosamax has been in use since September 29, 1995 after gaining Food and Drug Administration (FDA) approval. The drug obtained FDA clearance based on data from five clinical trials, lasting two years, involving 1,827 postmenopausal women with osteoporosis in 16 different countries. The trials showed a significant increase in bone mineral density at the spine, hip, and other sites. Overall, the drug reduced the number of women with new spinal fractures by 48%, the total number of new spinal fractures by 63%, and reduced overall height loss by 35%. Today, the drug remains in circulation in caplet and liquid forms and attainable at most pharmaceutical distribution centers with an eligible prescription.
Side Effects [4]
Common side effects of Fosamax include gas, constipation, heartburn, diarrhea, bloating, nausea, vomiting, stomach pain, joint pain or swelling, swelling in your hands or feet, dizziness, headache, eye pain, back pain, or weakness. Serious [but rare] side effects of Fosamax include severe pain (joints, bone, muscle, jaw, back, or heartburn), difficulty swallowing, bloody stools, eye pain, skin blisters, and swelling of the face, tongue, or throat.
Structural Highlights [5]
As a bisphosphonate, Fosamax is a synthetic analog of pyrophosphate, containing two phosphate groups on the central carbon. In its drug form, Fosamax includes a sodium bounded in place of a carboxyl hydrogen. In solution, oxyanions may form an ionic bond(s) with metal cations, such as magnesium. The central carbon is also attached to a hydroxyl group (-OH) and a three-carbon chain [-(CH2)3] leading to an amino group (-NH2) (Fig. 2).
| |||||||||||
Mechanism of Action
Functioning as a nitrogen-containing, second generation bisphosphonate, Fosamax binds to hydroxyapatite in bone and promotes apoptosis in osteoclasts (cells specialized in skeletal breakdown) (Fig. 6), thus slowing the breakdown of osseous tissue[7]. In order to accomplish this, Fosamax inhibits farnesyl pyrophosphate synthase (FPPS), an enzyme which plays a role within the mevalonic acid pathway, thereby reducing the production rate of isoprenoid compounds that are essential for post-translational modification of small guanosine triphosphate (GTP)-binding proteins (e.g. Rho, Rab and Ras)[8]. By inhibiting FPPS, Fosamax discourages the successive condensation of isopentenyl pyrophosphate with dimethylallyl pyrophosphate to geranyl pyrophosphate, which interferes with osteoclast function and survival [9][10].
Though the exact chemical mechanism by which alendronate inhibits FPPS remains unclear, nitrogen-containing bisphosphates (s) bind to the dimethylallyl/geranyl pyrophosphate (DMAPP/GPP) ligand pocket (Fig. 3) and alter the secondary structure of the enzyme[12]. Specifically, alendronate may bind to the FPPS allylic substrate-binding pocket by ligating the Magnesium ion center via phosphonate oxyanion nucleophilic attack[13]. Phosphonate groups are stabilized by a cluster of three Mg2+ ions via oxyanion-metal cation charge interactions, as well as three FPPS-conserved aspartate residues located within two alpha helical motifs () that form a (Fig. 4)[14]. N-BPs may bind to the GPP substrate-binding site because N-BPs resemble the structure of the enzyme’s natural substrates (GPP and DMAPP) and act as carbocation transition state analogs (Fig. 5). Interactions between N-BP and amino acid residues () suggest that these inhibitors in the carbocation-binding site, then mimic the carbocation intermediate formed via substrate ionization [15]. By acting as a transition state analog for isoprenoid biosynthesis, Fosamax effectively increases the activation energy of a regulatory/rate-determining step in the mevalonate pathway, subsequently reducing the concentration of product molecules that aid the continuous biodegradation of skeletal tissue.
References
- ↑ Gong Li, Altman Russ B, Klein Teri E . "Bisphosphonates pathway" Pharmacogenetics and genomics (2011). from https://www.pharmgkb.org/pathway/PA154423660
- ↑ Fosamax: Uses, Dosage & Side Effects - Drugs.com. (2016, November 3).Fosamax: Uses, Dosage & Side Effects - Drugs.com. Retrieved November 14, 2016, from https://www.drugs.com/fosamax.html
- ↑ Fosamax (alendronate sodium). Fosamax New FDA Drug Approval | CenterWatch. Merck. Retrieved November 15, 2016, from http://www.centerwatch.com/drug-information/fda-approved-drugs/drug/26/fosamax-alendronate-sodium
- ↑ Common Side Effects of Fosamax (Alendronate Sodium) Drug Center - RxList. (2015, August 26). RxList. Retrieved November 14, 2016, from http://www.rxlist.com/fosamax-side-effects-drug-center.htm
- ↑ Protein Data Bank in Europe: Bringing Structure to Biology, from http://www.ebi.ac.uk/pdbe/entry/pdb/2F89
- ↑ Martin, M. B., Arnold, W., Heath, H. T., III, Urbina, J. A., and Oldfield, E. (1999) Biochem. Biophys. Res. Commun. 263, 754–758
- ↑ Drake, Matthew T., Bart L. Clarke, and Sundeep Khosla. "Bisphosphonates: Mechanism of Action and Role in Clinical Practice." Mayo Clinic Proceedings 83, no. 9 (2008): 1032-045. doi:10.4065/83.9.1032.
- ↑ Luckman SP, Hughes DE, Coxon FP, Russell RGG, Rogers MJ (2005). Nitrogen-Containing Biphosphonates Inhibit the Mevalonate Pathway and Prevent Post-Translational Prenylation of GTP-Binding Proteins, Including Ras. Journal of Bone and Mineral Research. 20, 1265–74
- ↑ Alendronic acid. (2005, June 13), from http://www.drugbank.ca/drugs/DB00630#bond-15126
- ↑ Fisher, J. E., Rogers, M. J., Halasy, J. M., Luckman, S. P., Hughes, D. E., Masarachia, P. J., … Reszka, A. A. (1999). Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proceedings of the National Academy of Sciences, 96(1), 133–138. doi:10.1073/pnas.96.1.133, from http://www.pnas.org/content/96/1/133.full.pdf
- ↑ Martin, M. B., Arnold, W., Heath, H. T., III, Urbina, J. A., and Oldfield, E. (1999) Biochem. Biophys. Res. Commun. 263, 754–758
- ↑ Kavanagh KL, Guo K, Dunford JE, Wu X, Knapp S, and Ebetino FH (2006). The molecular mechanism of nitrogen-containing bisphosphonates as antiosteoporosis drugs. Proceedings of the National Academy of Sciences. 103, 7829–34
- ↑ Hosfield DJ, Zhang Y, Dougan DR, Broun A, Tari LW, Swanson RV (2003). Structural Basis for Bisphosphonate-mediated Inhibition of Isoprenoid Biosynthesis. Journal of Biological Chemistry. 2279, 8526–9
- ↑ Tarshis L. C., Yan M., Poulter C. D., Sacchettini J. C. (1994) Biochemistry. 33, 10871–10877.
- ↑ Martin, M. B., Arnold, W., Heath, H. T., III, Urbina, J. A., and Oldfield, E. (1999) Biochem. Biophys. Res. Commun. 263, 754–758
Contributors and Editors
Floro, Alyssa Jewel D. Kidd, Justin M. Samady, Osna M.