This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Rebetol
From Proteopedia
(Difference between revisions)
(New page: (Ribavirin) 1-β-D-ribofuranosyl-1H-1,2,4-triazole-3-carboxamide <ref name="gish">DOI: 10.1093/jac/dki405 </ref> <StructureSection load='' size='340' side='right' caption='Ribavirin PDB [[...) |
|||
| (7 intermediate revisions not shown.) | |||
| Line 2: | Line 2: | ||
<StructureSection load='' size='340' side='right' caption='Ribavirin PDB [[4pb1]]' scene='74/746008/Ribavirin_atalla2/1'> | <StructureSection load='' size='340' side='right' caption='Ribavirin PDB [[4pb1]]' scene='74/746008/Ribavirin_atalla2/1'> | ||
== Overview == | == Overview == | ||
| - | Ribavirin was first synthesized in 1970 by ICN Pharmaceuticals (now “Valent International Pharmaceuticals”). In 1986, its first major use was the treatment of RSV (respiratory syncitial virus) infections in pediatric patients, but since its FDA approval in 1998, it has primarily been used as a component in treating Hepatitis C by inhibiting the synthesis of viral RNA. <ref>https://pubchem.ncbi.nlm.nih.gov/compound/37542<ref> The treatment was modified and approved in 2002 by the FDA by combining | + | Ribavirin was first synthesized in 1970 by ICN Pharmaceuticals (now “Valent International Pharmaceuticals”). In 1986, its first major use was the treatment of RSV (respiratory syncitial virus) infections in pediatric patients, but since its FDA approval in 1998, it has primarily been used as a component in treating Hepatitis C by inhibiting the synthesis of viral RNA. <ref>https://pubchem.ncbi.nlm.nih.gov/compound/37542<ref> The treatment for these infections was modified and approved in 2002 by the FDA by combining Ribavirin with interferon alfa2b. <ref name="gish"/> |
== Structure and Function == | == Structure and Function == | ||
| - | Sentence | + | Sentence |
== Structure and Function == | == Structure and Function == | ||
| Line 11: | Line 11: | ||
== Structure and Function == | == Structure and Function == | ||
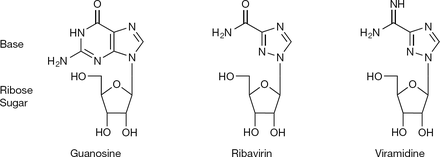
| - | While the main function of Ribavirin is to treat Hepatitis C and RSV, Ribavirin alone is not enough to treat these diseases and is commonly combined with interferon alpha2b<ref name="gish"/>. Ribavirin contains antiviral activity which inhibits DNA/RNA synthesis. It is chemically classified as a nucleoside analog because the structure of Ribavirin resembles the structure of the nucleoside guanosine. Ribavirin is water soluble and is able to mimic other purines, | + | While the main function of Ribavirin is to treat Hepatitis C and RSV, Ribavirin alone is not enough to treat these diseases and is commonly combined with interferon alpha2b<ref name="gish"/>. Ribavirin contains antiviral activity which inhibits DNA/RNA synthesis. It is chemically classified as a nucleoside analog because the structure of Ribavirin resembles the structure of the nucleoside guanosine. Ribavirin is water soluble and is able to mimic other purines, such as adenosine. However, a key difference between the structure of Ribavirin and the purine nucleosides is that the cyclic attachment to the ribose in Ribavirin contains only one ring, as opposed to purines which are heterocyclic. Despite this, it is able to go through similar mechanisms as that of nucleosides, such as phosphorylating into a triphosphate. Its structural similarity to the common nucleoside guanosine may suggest how the drug can inhibit DNA/RNA synthesis through purine mimicry. <ref name="structure"> doi: 10.3851/IMP2125 </ref> |
<scene name='74/746008/Ribavirin_atalla2/1'>Ribavirin</scene> [[4pb1]] | <scene name='74/746008/Ribavirin_atalla2/1'>Ribavirin</scene> [[4pb1]] | ||
| Line 17: | Line 17: | ||
[[Image:Ribavirin Structure1.gif]] | [[Image:Ribavirin Structure1.gif]] | ||
| + | |||
Comparison of the 2D structure of ribavirin to the structures of the nucleoside guanosine and a similiar anti-viral drug viramidine. <ref name= "structure"/> | Comparison of the 2D structure of ribavirin to the structures of the nucleoside guanosine and a similiar anti-viral drug viramidine. <ref name= "structure"/> | ||
== Protein Interaction == | == Protein Interaction == | ||
| - | ===Ribavirin with | + | ===Ribavirin with RNA-dependent RNA polymerase === |
Virally-encoded RNA-dependent RNA polymerase (RdRp) is critical for successful viral replication. The incorporation of nucleoside analogs, such as Ribavirin, by RdRp have been shown to induce error in viral replication | Virally-encoded RNA-dependent RNA polymerase (RdRp) is critical for successful viral replication. The incorporation of nucleoside analogs, such as Ribavirin, by RdRp have been shown to induce error in viral replication | ||
| - | Ribavirin attaches to the binding pocket, which is comprised of tyrosine at position 344 and aspartic acid at positions 250, 346, and 347. The incorporation of Ribavirin into the RNA results in base-pairing with uracil or cytosine, which increases the mutation rate and leads to viral death. | + | Ribavirin attaches to the binding pocket, which is comprised of tyrosine at position 344 and aspartic acid at positions 250, 346, and 347. The incorporation of Ribavirin into the RNA results in base-pairing with uracil or cytosine, which increases the mutation rate and leads to viral death. <ref>http://dx.doi.org/10.1016/j.virol.2012.01.016</ref> |
<scene name='55/559112/Ribavirin_interacting_3sfu/4'>Ribavirin with 3SFU</scene> [[3sfu]] | <scene name='55/559112/Ribavirin_interacting_3sfu/4'>Ribavirin with 3SFU</scene> [[3sfu]] | ||
| - | === Ribavirin with | + | === Ribavirin with CNT2=== |
Nucleoside transporters facilitate the transport of nucleosides across cell membranes to be used in protein synthesis. Concentrated NTs (CNTs) use the energy of ion gradients for active transportation , while equilibrative NTs (ENTs) transport nucleosides passively down their concentration gradient. Ribavirin is a substrate of ENT1, ENT2, and CNT2. CNT2 binds to the ribose of the ribavirin via hydrogen bonding involving glutamic acid at position 332, asparagine at position 368, and serine at position 371<ref>doi:10.7554/eLife.03604</ref>. | Nucleoside transporters facilitate the transport of nucleosides across cell membranes to be used in protein synthesis. Concentrated NTs (CNTs) use the energy of ion gradients for active transportation , while equilibrative NTs (ENTs) transport nucleosides passively down their concentration gradient. Ribavirin is a substrate of ENT1, ENT2, and CNT2. CNT2 binds to the ribose of the ribavirin via hydrogen bonding involving glutamic acid at position 332, asparagine at position 368, and serine at position 371<ref>doi:10.7554/eLife.03604</ref>. | ||
<scene name='55/559112/Ribavirin_interacting_4pb1/1'>Ribivirin with 4PB1</scene> [[4pb1]] | <scene name='55/559112/Ribavirin_interacting_4pb1/1'>Ribivirin with 4PB1</scene> [[4pb1]] | ||
| Line 59: | Line 60: | ||
# Thomas, E., Ghany, M., Liang, J., The application and mechanism of action of ribavirin in therapy of hepatitis C. Antiviral Chemistry & Chemotherapy 23:1-12 (2012)doi: 10.3851/IMP2125 | # Thomas, E., Ghany, M., Liang, J., The application and mechanism of action of ribavirin in therapy of hepatitis C. Antiviral Chemistry & Chemotherapy 23:1-12 (2012)doi: 10.3851/IMP2125 | ||
# Chung, R.T., Gale, M.J., Polyak, S.J., Lemon, S.M., Liang, T.J., & Hoofnagle, J.H. Mechanisms of action of interferon and ribavirin in chronic hepatitis C: Summary of a workshop. Hepatology. 2008;47 (1), 306-320. doi: 10.1002/hep.22070 | # Chung, R.T., Gale, M.J., Polyak, S.J., Lemon, S.M., Liang, T.J., & Hoofnagle, J.H. Mechanisms of action of interferon and ribavirin in chronic hepatitis C: Summary of a workshop. Hepatology. 2008;47 (1), 306-320. doi: 10.1002/hep.22070 | ||
| + | #Alam, I., Lee, J., Cho, K. J., Han, K.R., Yang, J.M., Chung, M.S., & Kim, K. H. Crystal structures of murine norovirus-1 RNA-dependent RNA polymerase in complex with 2-thiouridine or ribavirin. Virology. 2012, 426: 143-151. http://dx.doi.org/10.1016/j.virol.2012.01.016 | ||
| + | #Johnson, ZL, Lee, JH, Lee, M, Kwon, DY, Hong, J, Lee, SY. Structural basis of nucleoside and nucleoside drug selectivity by concentrative nucleoside transporters. eLife. 2014, 3:e03604. doi: 10.7554/eLife.03604. | ||
# Paeshuyse, J, Dallmeier, K, Neyts, J. Ribavirin for the treatment of chronic hepatitis C virus infection: a review of the proposed mechanisms of action. Current Opinion in Virology. 2011;1(6) 590-598. doi: 10.1016/j.coviro.2011.10.030 | # Paeshuyse, J, Dallmeier, K, Neyts, J. Ribavirin for the treatment of chronic hepatitis C virus infection: a review of the proposed mechanisms of action. Current Opinion in Virology. 2011;1(6) 590-598. doi: 10.1016/j.coviro.2011.10.030 | ||
# National Heart, Lung and Blood Institute. Pneumonia. 2016, September 26; Retrieved from https://www.nhlbi.nih.gov/health/health-topics/topics/pnu | # National Heart, Lung and Blood Institute. Pneumonia. 2016, September 26; Retrieved from https://www.nhlbi.nih.gov/health/health-topics/topics/pnu | ||
# Foster, G. Pegylated interferons for the treatment of chronic Hepatitis C. Drugs. 2010;70(2):147-165. doi:10.2165/11531990-000000000-00000 | # Foster, G. Pegylated interferons for the treatment of chronic Hepatitis C. Drugs. 2010;70(2):147-165. doi:10.2165/11531990-000000000-00000 | ||
#Hofmann WP, Herrmann E, Sarrazin C, Zeuzem S. Ribavirin mode of action in chronic hepatitis C: from clinical use back to molecular mechanisms. Liver Int. 2008, 28:1332-1343. doi: 10.1111/j.1478-3231.2008.01896.x | #Hofmann WP, Herrmann E, Sarrazin C, Zeuzem S. Ribavirin mode of action in chronic hepatitis C: from clinical use back to molecular mechanisms. Liver Int. 2008, 28:1332-1343. doi: 10.1111/j.1478-3231.2008.01896.x | ||
| - | #Alam, I., Lee, J., Cho, K. J., Han, K.R., Yang, J.M., Chung, M.S., & Kim, K. H. Crystal structures of murine norovirus-1 RNA-dependent RNA polymerase in complex with 2-thiouridine or ribavirin. Virology. 2012, 426: 143-151. http://dx.doi.org/10.1016/j.virol.2012.01.016 | ||
| - | #Johnson, ZL, Lee, JH, Lee, M, Kwon, DY, Hong, J, Lee, SY. Structural basis of nucleoside and nucleoside drug selectivity by concentrative nucleoside transporters. eLife. 2014, 3:e03604. doi: 10.7554/eLife.03604. | ||
#Fujimoto T, Tomimatsu M, Iga D, Endo H, Otsuka K. Changes in the Th1/Th2 ratio during a 24-week course of an interferon alpha-2b plus ribavirin combination therapy for patients with chronic hepatitis C. J. Gastroenterol. Hepatol. 2008, 23:E432- E437. doi: 10.1111/j.1440-1746.2008.05320.x | #Fujimoto T, Tomimatsu M, Iga D, Endo H, Otsuka K. Changes in the Th1/Th2 ratio during a 24-week course of an interferon alpha-2b plus ribavirin combination therapy for patients with chronic hepatitis C. J. Gastroenterol. Hepatol. 2008, 23:E432- E437. doi: 10.1111/j.1440-1746.2008.05320.x | ||
#Feld, JJ, Nanda, S, Huang, Y, Chen, W, Cam, M, Pusek, SN, Schwigler, LM, Theodore, D, Zacks, SL, Liang, TJ, Fried, MW. Hepatic gene expression during treatment with peginterferon and ribavirin: Identifying molecule pathways for treatment response. Hepatol. 2007, 46(5): 1548-1563. doi: 10.1002/hep.21853 | #Feld, JJ, Nanda, S, Huang, Y, Chen, W, Cam, M, Pusek, SN, Schwigler, LM, Theodore, D, Zacks, SL, Liang, TJ, Fried, MW. Hepatic gene expression during treatment with peginterferon and ribavirin: Identifying molecule pathways for treatment response. Hepatol. 2007, 46(5): 1548-1563. doi: 10.1002/hep.21853 | ||
Current revision
(Ribavirin) 1-β-D-ribofuranosyl-1H-1,2,4-triazole-3-carboxamide [1]
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Taylor H. Derby, Jamie Costa, Shannon Shaughnessy, Edmond R Atalla, Katherine Reynolds