User:Charli Barbet/Sandbox
From Proteopedia
< User:Charli Barbet(Difference between revisions)
| (7 intermediate revisions not shown.) | |||
| Line 14: | Line 14: | ||
<scene name='75/750264/Sh2/1'>SH2 DOMAIN</scene>: | <scene name='75/750264/Sh2/1'>SH2 DOMAIN</scene>: | ||
| - | SH2 domain is a domain that is approximately 100 amino acids long with a very conserved structure. | + | SH2 domain is a domain that is approximately 100 amino acids long with a very conserved structure. It has been identified in several human and rodent proteins such as [https://en.wikipedia.org/wiki/Phosphatase phosphatases], [https://en.wikipedia.org/wiki/Transcription_factor transcription factor], or [https://en.wikipedia.org/wiki/Signal_transducing_adaptor_protein adaptor] protein like Grb2. |
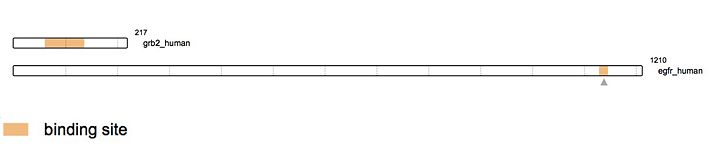
This domain is ubiquitous in several protein implicated in cellular signaling pathways.Typically, the SH2 domain specifically recognizes sites with '''phosphorylated tyrosines'''. SH2 can, for instance bind to the intracellular region of EGF leading in turn, to the formation of protein signalization complexes. This binding and the role of SH2 is very important in '''the conversion of an extra-cellular signal in an intra-cellular signal''' giving rise to diversified cellular responses or the expression of specific genes. It is also important to note that the SH2 domain can bind to other SH2 domains. Nevertheless, a mutation in the specific binding site of SH2 can impede the interaction of two proteins and thus the formation of a protein complex. Therefore, mutations in SH2 can give rise to cellular dysfunction and lead to several diseases. <ref>PMID: 18767163</ref> | This domain is ubiquitous in several protein implicated in cellular signaling pathways.Typically, the SH2 domain specifically recognizes sites with '''phosphorylated tyrosines'''. SH2 can, for instance bind to the intracellular region of EGF leading in turn, to the formation of protein signalization complexes. This binding and the role of SH2 is very important in '''the conversion of an extra-cellular signal in an intra-cellular signal''' giving rise to diversified cellular responses or the expression of specific genes. It is also important to note that the SH2 domain can bind to other SH2 domains. Nevertheless, a mutation in the specific binding site of SH2 can impede the interaction of two proteins and thus the formation of a protein complex. Therefore, mutations in SH2 can give rise to cellular dysfunction and lead to several diseases. <ref>PMID: 18767163</ref> | ||
<scene name='75/750264/Sh3/1'>SH3 DOMAIN</scene>: | <scene name='75/750264/Sh3/1'>SH3 DOMAIN</scene>: | ||
| - | The SH3 domain is approximately 50 amino acid long. Largely '''expressed in proteins associated with the membrane'''. The domain is made of 5 to 6 β-sheets arranged in two antiparallel β-sheets. The linking region between the two β-sheets is made of α helices. This special conformation allows the '''arrangement of a hydrophobic pocket in which the ligand can bind.''' Typically, the binding region has a '''motif rich in Prolines: PXXP'''. This binding allows the formation of multi- | + | The SH3 domain is approximately 50 amino acid long. Largely '''expressed in proteins associated with the membrane'''. The domain is made of 5 to 6 β-sheets arranged in two antiparallel β-sheets. The linking region between the two β-sheets is made of α helices. This special conformation allows the '''arrangement of a hydrophobic pocket in which the ligand can bind.''' Typically, the binding region has a '''motif rich in Prolines: PXXP'''. This binding allows the formation of multi-protein complexes involved in the translation and conversion of extra-cellular signals. The binding is thus largely involved in gene expression and protein concentration. <ref>PMID: 1279434</ref> |
'''ISOFORM''': | '''ISOFORM''': | ||
Grb2 posses an isoform, known as '''Grb3.3'''. | Grb2 posses an isoform, known as '''Grb3.3'''. | ||
| - | Grb3.3 is present in cells but it '''induces apoptosis'''. The isoform has a very similar structure to Grb2 but is truncated from | + | Grb3.3 is present in cells but it '''induces apoptosis'''. The isoform has a very similar structure to Grb2 but is truncated from the 60th to the 100th amino acid resulting in a degradation of the SH2 domain and a loss of functionality. <ref>PMID: 8178156</ref> |
== '''Function''' == | == '''Function''' == | ||
| - | The | + | The Grb3.3 isoform has a non-functional SH2 domain, unable to bind the phosphorylated tyrosines of its targeted protein (EGFR for instance). The inability of the molecule to transmit signal is translated by apoptosis of the cell, thus regulating growth signal. |
The functional isoform: Grb2, is involved in several cellular functions detailed below: | The functional isoform: Grb2, is involved in several cellular functions detailed below: | ||
| Line 42: | Line 42: | ||
As an example, the phosphorylated residues of [http://www.uniprot.org/uniprot/O43561 LAT] can bind the SH2 domain of Grb2 while the formation of this complex recruits on the SH3 domains of Grb2 some proteins of the [https://en.wikipedia.org/wiki/Vav_(protein) VAV family]. [http://www.uniprot.org/uniprot/P15498 '''VAV proteins] are guanine nucleotide exchange factors''' ([https://en.wikipedia.org/wiki/Guanine_nucleotide_exchange_factor GEF]) for the [https://en.wikipedia.org/wiki/GTPase GTPase proteins] of the [https://en.wikipedia.org/wiki/Rho_family_of_GTPases Rho family]. The formation of this specific complex introduces a Calcium flux and activates [https://en.wikipedia.org/wiki/Mitogen-activated_protein_kinase MAP kinase] allowing [https://en.wikipedia.org/wiki/T_cell T lymphocyte] proliferation.<ref> PMID 15886116</ref> | As an example, the phosphorylated residues of [http://www.uniprot.org/uniprot/O43561 LAT] can bind the SH2 domain of Grb2 while the formation of this complex recruits on the SH3 domains of Grb2 some proteins of the [https://en.wikipedia.org/wiki/Vav_(protein) VAV family]. [http://www.uniprot.org/uniprot/P15498 '''VAV proteins] are guanine nucleotide exchange factors''' ([https://en.wikipedia.org/wiki/Guanine_nucleotide_exchange_factor GEF]) for the [https://en.wikipedia.org/wiki/GTPase GTPase proteins] of the [https://en.wikipedia.org/wiki/Rho_family_of_GTPases Rho family]. The formation of this specific complex introduces a Calcium flux and activates [https://en.wikipedia.org/wiki/Mitogen-activated_protein_kinase MAP kinase] allowing [https://en.wikipedia.org/wiki/T_cell T lymphocyte] proliferation.<ref> PMID 15886116</ref> | ||
| - | Finally, it was proven that Grb2 plays a role in '''the negative regulation of''' [http://www.uniprot.org/uniprot/P00533''' EGFR]'''. Indeed, [http://www.uniprot.org/uniprot/P22681 c-Cbl] is a protein implicated in the [http://www.uniprot.org/uniprot/O60260 '''E3] complex of''' [http://www.uniprot.org/uniprot/P00533''' EGFR] ubiquitination'''.[http://www.uniprot.org/uniprot/P22681 C-Cbl] thanks to its SH2 domain can directly bind to [http://www.uniprot.org/uniprot/P00533 EGFR] causing its degradation (Grb2 independent regulation). | + | Finally, it was proven that Grb2 plays a role in '''the negative regulation of''' [http://www.uniprot.org/uniprot/P00533''' EGFR]'''. Indeed, [http://www.uniprot.org/uniprot/P22681 c-Cbl] is a protein implicated in the [http://www.uniprot.org/uniprot/O60260 '''E3] complex of''' [http://www.uniprot.org/uniprot/P00533''' EGFR] ubiquitination'''.[http://www.uniprot.org/uniprot/P22681 C-Cbl] thanks to its SH2 domain can directly bind to [http://www.uniprot.org/uniprot/P00533 EGFR] causing its degradation (Grb2 independent regulation). Or, [http://www.uniprot.org/uniprot/P22681 c-Cbl] can also indirectly bind to [http://www.uniprot.org/uniprot/P00533 EGFR] via its SH3 domain recognition by Grb2 (dependant Grb2 regulation). The direct or indirect binding of [http://www.uniprot.org/uniprot/P22681 c-Cbl] on [http://www.uniprot.org/uniprot/P00533 EGFR] induces the recruitment of enzymes that are necessary for the ubiquitination of [http://www.uniprot.org/uniprot/P00533 EGFR]. Ubiquitination being a signal for protein degradation. It is important to note that n'''egative regulation is more important when Grb2 is implicated and bound to''' [http://www.uniprot.org/uniprot/P22681 '''c-Cbl] rather than when''' [http://www.uniprot.org/uniprot/P22681 '''c-Cbl] is the only protein involved'''. <ref>PMID 10531381</ref> <ref> PMID 11823423</ref> |
| Line 57: | Line 57: | ||
[http://www.uniprot.org/uniprot/Q13094 '''LCP2''']: Involved in [https://en.wikipedia.org/wiki/T-cell_receptor T cell antigen receptor] mediated signaling. <ref>PMID: 10204582</ref> | [http://www.uniprot.org/uniprot/Q13094 '''LCP2''']: Involved in [https://en.wikipedia.org/wiki/T-cell_receptor T cell antigen receptor] mediated signaling. <ref>PMID: 10204582</ref> | ||
| - | [http://www.uniprot.org/uniprot/P04626 '''Erbb2''']: [http://www.uniprot.org/uniprot/P04626 Erbb2] is a kinase involved in several surface receptor complexes, but need a co-receptor for ligand binding. For example, it participates in neuregulin receptor complex but it can’t bind | + | [http://www.uniprot.org/uniprot/P04626 '''Erbb2''']: [http://www.uniprot.org/uniprot/P04626 Erbb2] is a kinase involved in several surface receptor complexes, but need a co-receptor for ligand binding. For example, it participates in neuregulin receptor complex but it can’t bind to it on its own. <ref>PMID: 16729043</ref> |
[http://www.uniprot.org/uniprot/Q8WU20 '''Frs2''']: [http://www.uniprot.org/uniprot/P21802 Fibroblast growth factor receptor substrate 2] can bind to [http://www.uniprot.org/uniprot/P09769 FGR] and NGF activated receptor. They play an important role in the activation of MAPK kinase, or the phosphorylation of [http://www.uniprot.org/uniprot/P27986 PIK3R1]. <ref>PMID: 11997436</ref> | [http://www.uniprot.org/uniprot/Q8WU20 '''Frs2''']: [http://www.uniprot.org/uniprot/P21802 Fibroblast growth factor receptor substrate 2] can bind to [http://www.uniprot.org/uniprot/P09769 FGR] and NGF activated receptor. They play an important role in the activation of MAPK kinase, or the phosphorylation of [http://www.uniprot.org/uniprot/P27986 PIK3R1]. <ref>PMID: 11997436</ref> | ||
| Line 89: | Line 89: | ||
[[Image:Y160.jpg|thumb|upright=3|[http://www.nature.com/articles/ncomms8354#abstract source]]] | [[Image:Y160.jpg|thumb|upright=3|[http://www.nature.com/articles/ncomms8354#abstract source]]] | ||
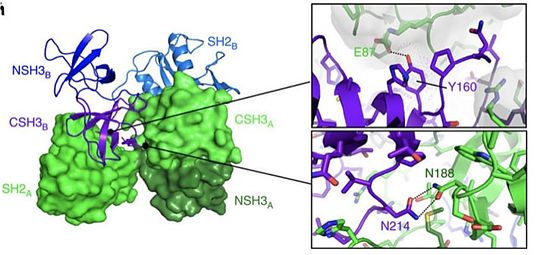
| - | Grb2 protein is especially involved in the '''setting up of cellular oncognesis''' in prostate, colon and lung cancers. This role is mainly due to its essential role in signal transduction in the [https://en.wikipedia.org/wiki/Mitogen-activated_protein_kinase MAP kinase pathway] known to induce [https://en.wikipedia.org/wiki/Mitosis mitosis]. In this pathway, Grb2 binds to the oncogenic protein [http://www.uniprot.org/uniprot/Q07889 SOS] under its monomeric form. Yet | + | Grb2 protein is especially involved in the '''setting up of cellular oncognesis''' in prostate, colon and lung cancers. This role is mainly due to its essential role in signal transduction in the [https://en.wikipedia.org/wiki/Mitogen-activated_protein_kinase MAP kinase pathway] known to induce [https://en.wikipedia.org/wiki/Mitosis mitosis]. In this pathway, Grb2 binds to the oncogenic protein [http://www.uniprot.org/uniprot/Q07889 SOS] under its monomeric form. Yet Grb2 can also be found in its dimeric form in the cell. Dimerization of Grb2 is dependent upon several factors like the phosphorylation of <scene name='75/750264/Y160/1'>tyrosine 160</scene> or the binding of ligand on the SH2 domain of the same protein. Mainly, phosphorylation induces the dissociation of the Grb2 dimer bringing about an increase in the MAP kinase pathway by the binding of [http://www.uniprot.org/uniprot/Q07889 SOS]. The phosphorylated state of <scene name='75/750264/Y160/1'>Y160</scene> has been discovered in several pre-metastatis cancers, highly suggesting that pY160 could be a oncogenic marker in humans. A new therapeutic strategy could therefore be considered by stabilizing Grb2 in its dimeric form. This could be achieved with a protein acting as an irreversible cross-link at the interface between the two units. <ref>PMID: 26103942</ref> |
<br style="clear:both" /> | <br style="clear:both" /> | ||
Current revision
Grb2 (1gri)
| |||||||||||