Epoxidase
From Proteopedia
(Difference between revisions)
| (12 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
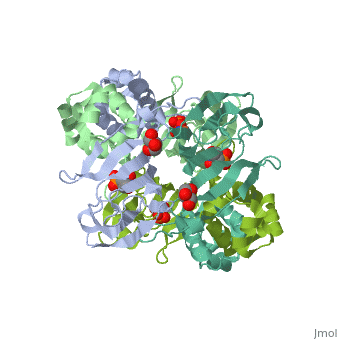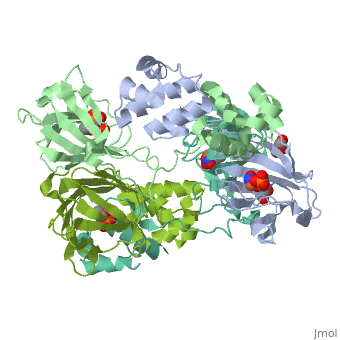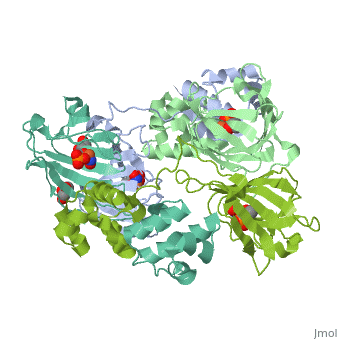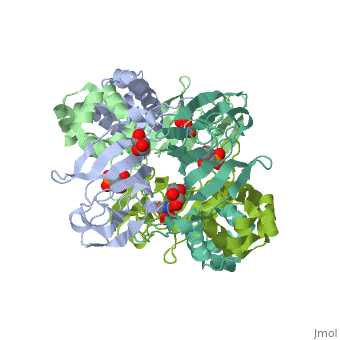
| - | <StructureSection load='3scf' size=' | + | <StructureSection load='3scf' size='350' side='right' caption='Hydroxypropylphosphonic acid epoxidase complex with fosfomycin, glycerol, NO and Fe+2 ion (PDB code [[3scf]])' scene='' pspeed='8'> |
== Function == | == Function == | ||
| - | '''Hydroxypropylphosphonic acid epoxidase''' (HppE) is involved in the biosynthesis of the antibiotic fosfomycin<ref>PMID:16186494</ref>. HppE is a Zn+2/Fe+2-dependent enzyme. | + | '''Hydroxypropylphosphonic acid epoxidase''' (HppE) is involved in the biosynthesis of the antibiotic fosfomycin<ref>PMID:16186494</ref>. HppE is a Zn+2/Fe+2-dependent enzyme. |
| + | *'''Halohydrin epoxidase''' (HHE) is involved in the degradation of halohydrins by the displacement of a halogen in halohydrins to produce the epoxide<ref>PMID:26422370</ref>. | ||
| + | *'''Squalene epoxidase''' or '''squalene monooxygenase''' is a rate-limiting enzyme in cholesterol biosynthesis<ref>PMID:30792392</ref>. | ||
| + | *'''1,2-phenylacetyl-CoA epoxidase''' is part of the catabolic pathway of Phe and phenylacetate<ref>PMID:20660314</ref>. | ||
== Relevance == | == Relevance == | ||
Fosfomycin is an antibiotic produced by some ''Streptomyces'' species. | Fosfomycin is an antibiotic produced by some ''Streptomyces'' species. | ||
| + | |||
| + | == Disease == | ||
| + | A single mutation in '''squalene epoxidase''' causes resistance to terbinafine, the antifungal treatment against dermatophytosis<ref>PMID:37236595</ref>. | ||
== Structural highlights == | == Structural highlights == | ||
| - | The active site of HppE contains fosfomycin and Fe+2. | + | The <scene name='75/752206/Cv/6'>active site of HppE contains fosfomycin and Fe+2</scene>. The enzyme uses an induced-fit mechanism to protect high-energy iron-oxygen species formed during catalysis by moving a <scene name='75/752206/Cv/7'>β-hairpin termed cantilever</scene> to seal off the top portion of the active site<ref>PMID:21682308</ref>. <scene name='75/752206/Cv/8'>Fe coordination site</scene>. |
| - | </StructureSection> | ||
==3D structures of epoxidase== | ==3D structures of epoxidase== | ||
| + | [[Epoxidase 3D structures]] | ||
| - | + | </StructureSection> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
== References == | == References == | ||
<references/> | <references/> | ||
| + | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
References
- ↑ McLuskey K, Cameron S, Hammerschmidt F, Hunter WN. Structure and reactivity of hydroxypropylphosphonic acid epoxidase in fosfomycin biosynthesis by a cation- and flavin-dependent mechanism. Proc Natl Acad Sci U S A. 2005 Oct 4;102(40):14221-6. Epub 2005 Sep 26. PMID:16186494
- ↑ Watanabe F, Yu F, Ohtaki A, Yamanaka Y, Noguchi K, Yohda M, Odaka M. Crystal structures of halohydrin hydrogen-halide-lyases from Corynebacterium sp. N-1074. Proteins. 2015 Dec;83(12):2230-9. doi: 10.1002/prot.24938. Epub 2015 Oct 16. PMID:26422370 doi:http://dx.doi.org/10.1002/prot.24938
- ↑ Brown AJ, Chua NK, Yan N. The shape of human squalene epoxidase expands the arsenal against cancer. Nat Commun. 2019 Feb 21;10(1):888. PMID:30792392 doi:10.1038/s41467-019-08866-y
- ↑ Teufel R, Mascaraque V, Ismail W, Voss M, Perera J, Eisenreich W, Haehnel W, Fuchs G. Bacterial phenylalanine and phenylacetate catabolic pathway revealed. Proc Natl Acad Sci U S A. 2010 Aug 10;107(32):14390-5. doi:, 10.1073/pnas.1005399107. Epub 2010 Jul 21. PMID:20660314 doi:10.1073/pnas.1005399107
- ↑ Gupta AK, Cooper EA, Wang T, Polla Ravi S, Lincoln SA, Piguet V, McCarthy LR, Bakotic WL. Detection of Squalene Epoxidase Mutations in United States Patients with Onychomycosis: Implications for Management. J Invest Dermatol. 2023 Dec;143(12):2476-2483.e7. PMID:37236595 doi:10.1016/j.jid.2023.04.032
- ↑ Yun D, Dey M, Higgins LJ, Yan F, Liu HW, Drennan CL. Structural Basis of Regiospecificity of a Mononuclear Iron Enzyme in Antibiotic Fosfomycin Biosynthesis. J Am Chem Soc. 2011 Jun 30. PMID:21682308 doi:10.1021/ja2025728