We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Connexin
From Proteopedia
(Difference between revisions)
| (12 intermediate revisions not shown.) | |||
| Line 4: | Line 4: | ||
Of notice, about half of all cases of human pre-lingual recessive deafness in countries surrounding the Mediterranean have been linked to mutations in the GJB2 gene<ref name='important'>pmid 24624091</ref>,<ref name='Structure'>pmid 19622859</ref> | Of notice, about half of all cases of human pre-lingual recessive deafness in countries surrounding the Mediterranean have been linked to mutations in the GJB2 gene<ref name='important'>pmid 24624091</ref>,<ref name='Structure'>pmid 19622859</ref> | ||
| + | *'''Connexin 26''' participates in K+ transport in sensory hair cells in the ear and its mutations are causes of deafness<ref>pmid 9285800</ref> | ||
| + | *'''Connexin 31''' mutations are associated with skin and auditory system disorders<ref>PMID 16549784</ref> | ||
| + | *'''Connexin 32''' is found in the peripheral nervous system and its mutations are associated with Charcot-Marie-Tooth disease<ref>PMID 30042657</ref> | ||
| + | *'''Connexin 36''' regulates the in vivo dynamics of insulin secretion which is important for glucose homeostasis<ref>PMID 22511206</ref> | ||
| + | *'''Connexin 40''' is found in the atrial myocardium and its mutations are associated with trial fibrillation<ref>PMID 19535379</ref> | ||
| + | *'''Connexin 43''' renders glioblastoma resistant to chemotherapy<ref>PMID 35022385</ref> | ||
| + | *'''Connexin 45''' is involved in the maturation of vessel<ref>PMID 10976050</ref> | ||
| + | *'''Connexin 46''' is found in the eye lens and is unregulated in human breast cancer<ref>PMID 32353936</ref> | ||
| + | *'''Connexin 50''' functions as adhesive molecule maintaining lens fibre integrity<ref>PMID 28706245</ref> | ||
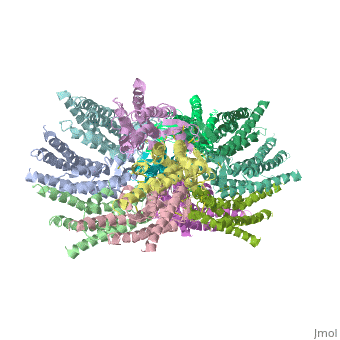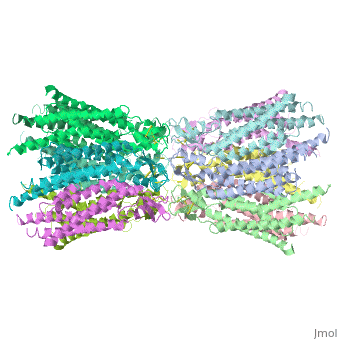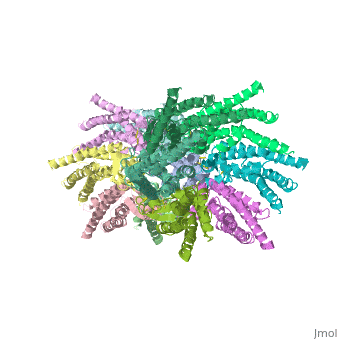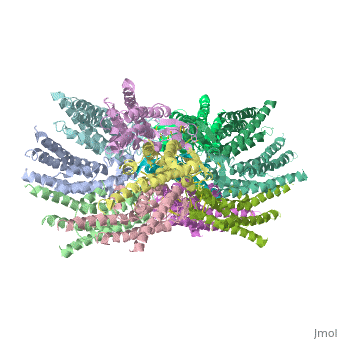
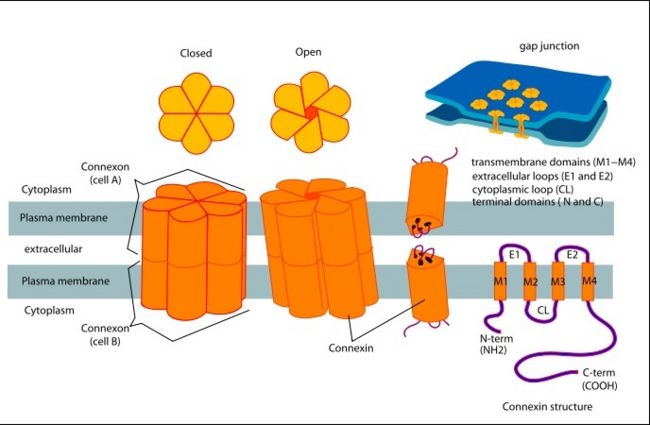
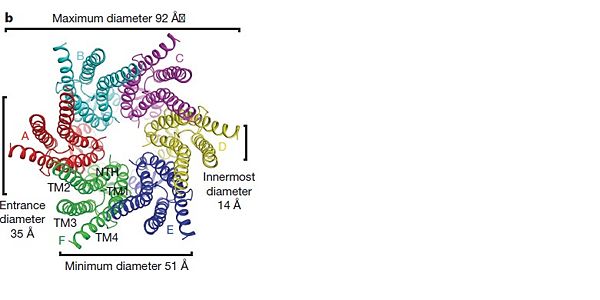
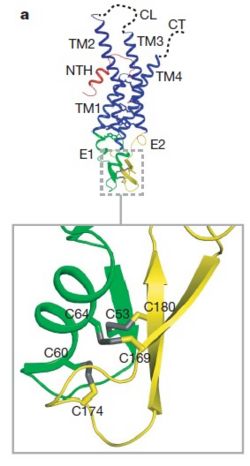
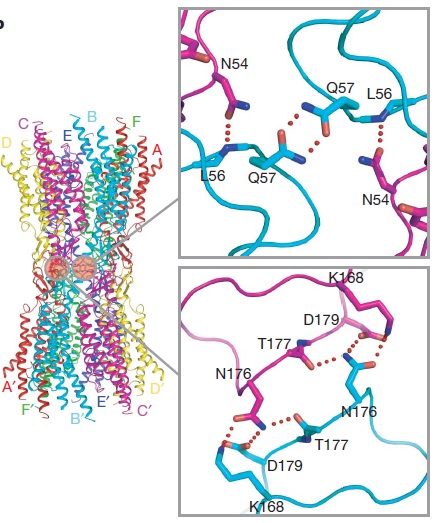
[[image:co.jpg | thumb |650px | center | The overall connexon's structure]] <ref>http://en.wikipedia.org/wiki/Connexin</ref> | [[image:co.jpg | thumb |650px | center | The overall connexon's structure]] <ref>http://en.wikipedia.org/wiki/Connexin</ref> | ||
| Line 42: | Line 51: | ||
The 3D structure of a mutant human connexin 26 <scene name='70/701426/Mutant_connexin26_-cx26m34a/1'>(Cx26M34A)</scene> channel shows an unexpected density within the vestibule of each hemichannel compared to the <scene name='70/701426/Wild_type_connexin/1'>wild type connexin 26</scene> , which is called a plug <ref name='pdb'/> , That plug was decreased in the the human mutant connexin 26 <scene name='70/701426/Deletion/1'>Cx26del2-7</scene> structure, indicating that the N terminus significantly contributes to form this plug feature. Experiments with this mutant show significantly reduced dye coupling between [http://en.wikipedia.org/wiki/HeLa HeLa cells] transiently expressing Cx26M34A gap junctions. <ref name='pdb'/> | The 3D structure of a mutant human connexin 26 <scene name='70/701426/Mutant_connexin26_-cx26m34a/1'>(Cx26M34A)</scene> channel shows an unexpected density within the vestibule of each hemichannel compared to the <scene name='70/701426/Wild_type_connexin/1'>wild type connexin 26</scene> , which is called a plug <ref name='pdb'/> , That plug was decreased in the the human mutant connexin 26 <scene name='70/701426/Deletion/1'>Cx26del2-7</scene> structure, indicating that the N terminus significantly contributes to form this plug feature. Experiments with this mutant show significantly reduced dye coupling between [http://en.wikipedia.org/wiki/HeLa HeLa cells] transiently expressing Cx26M34A gap junctions. <ref name='pdb'/> | ||
Functional analysis of the Cx26M34A channels revealed that these channels are predominantly closed, with the residual electrical conductance showing normal voltage gating. N-terminal deletion mutants with and without the M34A mutation showed no electrical activity in paired Xenopus oocytes and significantly decreased dye permeability in HeLa cells. Comparing this closed structure with the published X-ray structure of wild-type Cx26, which is proposed to be in an open state, revealed a radial outward shift in the transmembrane helices in the closed state, presumably to accommodate the N-terminal plug occluding the pore. Because both Cx26del2-7 and Cx26M34Adel2-7 channels are closed, the N terminus appears to have a prominent role in stabilizing the open configuration. <ref name='pdb'/> | Functional analysis of the Cx26M34A channels revealed that these channels are predominantly closed, with the residual electrical conductance showing normal voltage gating. N-terminal deletion mutants with and without the M34A mutation showed no electrical activity in paired Xenopus oocytes and significantly decreased dye permeability in HeLa cells. Comparing this closed structure with the published X-ray structure of wild-type Cx26, which is proposed to be in an open state, revealed a radial outward shift in the transmembrane helices in the closed state, presumably to accommodate the N-terminal plug occluding the pore. Because both Cx26del2-7 and Cx26M34Adel2-7 channels are closed, the N terminus appears to have a prominent role in stabilizing the open configuration. <ref name='pdb'/> | ||
| - | + | ||
=3D structures of connexin= | =3D structures of connexin= | ||
| + | [[Connexin 3D structure]] | ||
| - | + | </StructureSection> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
= References = | = References = | ||
<references/> | <references/> | ||
| + | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
References
- ↑ Zonta F, Buratto D, Cassini C, Bortolozzi M, Mammano F. Molecular dynamics simulations highlight structural and functional alterations in deafness-related M34T mutation of connexin 26. Front Physiol. 2014 Mar 4;5:85. doi: 10.3389/fphys.2014.00085. eCollection 2014. PMID:24624091 doi:http://dx.doi.org/10.3389/fphys.2014.00085
- ↑ 2.0 2.1 2.2 2.3 Suga M, Maeda S, Nakagawa S, Yamashita E, Tsukihara T. A description of the structural determination procedures of a gap junction channel at 3.5 A resolution. Acta Crystallogr D Biol Crystallogr. 2009 Aug;65(Pt 8):758-66. Epub 2009, Jul 10. PMID:19622859 doi:http://dx.doi.org/10.1107/S0907444909014711
- ↑ Zelante L, Gasparini P, Estivill X, Melchionda S, D'Agruma L, Govea N, Milá M, Monica MD, Lutfi J, Shohat M, Mansfield E, Delgrosso K, Rappaport E, Surrey S, Fortina P. Connexin26 mutations associated with the most common form of non-syndromic neurosensory autosomal recessive deafness (DFNB1) in Mediterraneans. Hum Mol Genet. 1997 Sep;6(9):1605-9. PMID:9285800 doi:10.1093/hmg/6.9.1605
- ↑ Abrams CK, Freidin MM, Verselis VK, Bargiello TA, Kelsell DP, Richard G, Bennett MV, Bukauskas FF. Properties of human connexin 31, which is implicated in hereditary dermatological disease and deafness. Proc Natl Acad Sci U S A. 2006 Mar 28;103(13):5213-8. PMID:16549784 doi:10.1073/pnas.0511091103
- ↑ Bortolozzi M. What's the Function of Connexin 32 in the Peripheral Nervous System? Front Mol Neurosci. 2018 Jul 10;11:227. PMID:30042657 doi:10.3389/fnmol.2018.00227
- ↑ Head WS, Orseth ML, Nunemaker CS, Satin LS, Piston DW, Benninger RK. Connexin-36 gap junctions regulate in vivo first secretion dynamics and glucose tolerance in the conscious mouse. Diabetes. 2012 Jul;61(7):1700-7. PMID:22511206 doi:10.2337/db11-1312
- ↑ Chaldoupi SM, Loh P, Hauer RN, de Bakker JM, van Rijen HV. The role of connexin40 in atrial fibrillation. Cardiovasc Res. 2009 Oct 1;84(1):15-23. PMID:19535379 doi:10.1093/cvr/cvp203
- ↑ Pridham KJ, Shah F, Hutchings KR, Sheng KL, Guo S, Liu M, Kanabur P, Lamouille S, Lewis G, Morales M, Jourdan J, Grek CL, Ghatnekar GG, Varghese R, Kelly DF, Gourdie RG, Sheng Z. Connexin 43 confers chemoresistance through activating PI3K. Oncogenesis. 2022 Jan 12;11(1):2. PMID:35022385 doi:10.1038/s41389-022-00378-7
- ↑ Krüger O, Plum A, Kim JS, Winterhager E, Maxeiner S, Hallas G, Kirchhoff S, Traub O, Lamers WH, Willecke K. Defective vascular development in connexin 45-deficient mice. Development. 2000 Oct;127(19):4179-93. PMID:10976050 doi:10.1242/dev.127.19.4179
- ↑ Acuña RA, Varas-Godoy M, Berthoud VM, Alfaro IE, Retamal MA. Connexin-46 Contained in Extracellular Vesicles Enhance Malignancy Features in Breast Cancer Cells. Biomolecules. 2020 Apr 28;10(5):676. PMID:32353936 doi:10.3390/biom10050676
- ↑ Hu Z, Shi W, Riquelme MA, Shi Q, Biswas S, Lo WK, White TW, Gu S, Jiang JX. Connexin 50 Functions as an Adhesive Molecule and Promotes Lens Cell Differentiation. Sci Rep. 2017 Jul 13;7(1):5298. PMID:28706245 doi:10.1038/s41598-017-05647-9
- ↑ http://en.wikipedia.org/wiki/Connexin
- ↑ Sokolov M, Brownstein Z, Frydman M, Avraham KB. Apparent phenotypic anticipation in autosomal dominant connexin 26 deafness. J Basic Clin Physiol Pharmacol. 2014 Sep;25(3):289-92. doi:, 10.1515/jbcpp-2014-0053. PMID:25153233 doi:http://dx.doi.org/10.1515/jbcpp-2014-0053
- ↑ 14.0 14.1 Ambrosi C, Walker AE, Depriest AD, Cone AC, Lu C, Badger J, Skerrett IM, Sosinsky GE. Analysis of trafficking, stability and function of human connexin 26 gap junction channels with deafness-causing mutations in the fourth transmembrane helix. PLoS One. 2013 Aug 15;8(8):e70916. doi: 10.1371/journal.pone.0070916. eCollection, 2013. PMID:23967136 doi:http://dx.doi.org/10.1371/journal.pone.0070916
- ↑ Kikuchi T, Adams JC, Miyabe Y, So E, Kobayashi T. Potassium ion recycling pathway via gap junction systems in the mammalian cochlea and its interruption in hereditary nonsyndromic deafness. Med Electron Microsc. 2000;33(2):51-6. PMID:11810458 doi:http://dx.doi.org/10.1007/s007950000009
- ↑ 16.0 16.1 16.2 16.3 16.4 Oshima A, Tani K, Toloue MM, Hiroaki Y, Smock A, Inukai S, Cone A, Nicholson BJ, Sosinsky GE, Fujiyoshi Y. Asymmetric Configurations and N-terminal Rearrangements in Connexin26 Gap Junction Channels. J Mol Biol. 2011 Jan 21;405(3):724-35. Epub 2010 Nov 20. PMID:21094651 doi:10.1016/j.jmb.2010.10.032
Proteopedia Page Contributors and Editors (what is this?)
Safaa Salah Hussiesy, Michal Harel, Doaa Naffaa, Jaime Prilusky