Vytorin
From Proteopedia
(Difference between revisions)
| (2 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | < | + | <StructureSection load='' size='340' side='right' caption='Ezetimibe and Simvastatin come together to make up the medication known as Vytorin' scene='75/757901/3d_structure_of_vytorin/1'> |
==Vytorin (ezetimibe & simvastatin)== | ==Vytorin (ezetimibe & simvastatin)== | ||
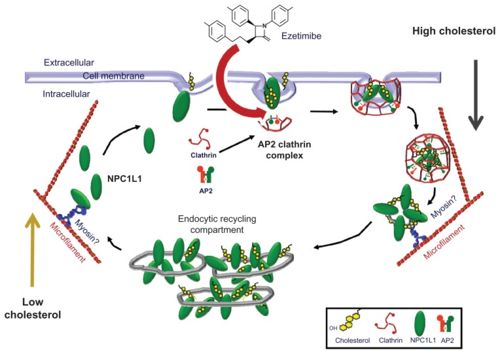
| - | Vytorin is a brand name drug used to treat Dyslipidemia, an abnormal amount of cholesterol or other lipids in the blood. It is composed of two separate medications, Simvastatin (generic for Zocor) and Ezetimibe (generic for Zetia). Ezetimibe works by decreasing cholesterol absorption in the small intestine. The second component of Vytorin, Simvastatin, works by reducing the production of cholesterol. | + | '''Vytorin''' is a brand name drug used to treat Dyslipidemia, an abnormal amount of cholesterol or other lipids in the blood. It is composed of two separate medications, Simvastatin (generic for Zocor) and Ezetimibe (generic for Zetia). Ezetimibe works by decreasing cholesterol absorption in the small intestine. The second component of Vytorin, Simvastatin, works by reducing the production of cholesterol. |
== Pharmacodynamics == | == Pharmacodynamics == | ||
| Line 27: | Line 27: | ||
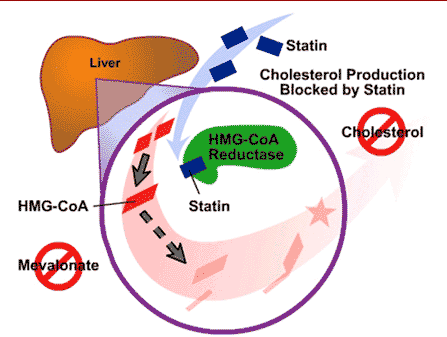
When the drug is administered, it is initially inactive. It must be hydrolyzed in order to become active. Once hydrolyzed, simvastatin reduces the amount of mevalonic acid in the blood by competing for HMG-CoA reductase with HMG-CoA. To produce cholesterol, Acetyl CoA is converted into HMG-CoA, which is then converted into mevalonate. Mevalonate is then converted into isopentenyl pyrophosphate (IPP), IPP is converted into squalene, and then finally squalene is converted into cholesterol. Simvastatin reduces the conversion of HMG-CoA to mevalonate, therefore lowering cholesterol levels. Simvastatin targets <scene name='75/758993/Simvastatin_bound/1'>HMG-CoA reductase</scene> which is the enzyme responsible for said conversion. With the synthesis of cholesterol being divided into five major steps, this inability to convert targets only the second step in cholesterol synthesis.<ref>http://www.merck.com/product/usa/pi_circulars/v/vytorin/vytorin_pi.pdf</ref> | When the drug is administered, it is initially inactive. It must be hydrolyzed in order to become active. Once hydrolyzed, simvastatin reduces the amount of mevalonic acid in the blood by competing for HMG-CoA reductase with HMG-CoA. To produce cholesterol, Acetyl CoA is converted into HMG-CoA, which is then converted into mevalonate. Mevalonate is then converted into isopentenyl pyrophosphate (IPP), IPP is converted into squalene, and then finally squalene is converted into cholesterol. Simvastatin reduces the conversion of HMG-CoA to mevalonate, therefore lowering cholesterol levels. Simvastatin targets <scene name='75/758993/Simvastatin_bound/1'>HMG-CoA reductase</scene> which is the enzyme responsible for said conversion. With the synthesis of cholesterol being divided into five major steps, this inability to convert targets only the second step in cholesterol synthesis.<ref>http://www.merck.com/product/usa/pi_circulars/v/vytorin/vytorin_pi.pdf</ref> | ||
| - | |||
| - | |||
| - | |||
| - | |||
---- | ---- | ||
| - | + | </StructureSection> | |
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
| |||||||||||
References
- ↑ https://www.drugbank.ca/drugs/DB00973
- ↑ https://pubchem.ncbi.nlm.nih.gov/compound/Ezetimibe#section=Experimental-Properties
- ↑ http://e-lactancia.org/media/papers/StatinasFK-FundClinPhar2004.pdf
- ↑ https://www.rpi.edu/dept/bcbp/molbiochem/MBWeb/mb2/part1/cholesterol.htm==Mechanism of Action
- ↑ https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021445s019lbl.pdf
- ↑ https://www.ncbi.nlm.nih.gov/pubmed/18522832
- ↑ http://www.merck.com/product/usa/pi_circulars/v/vytorin/vytorin_pi.pdf