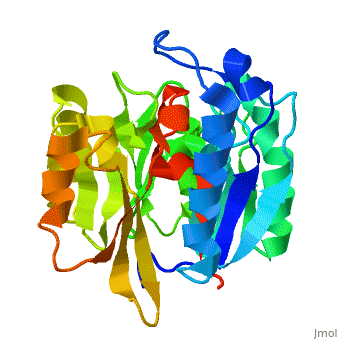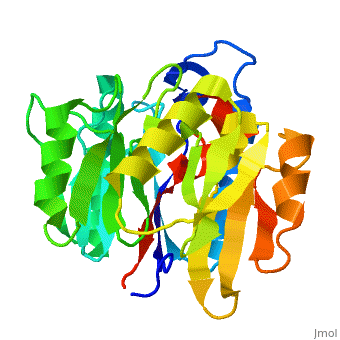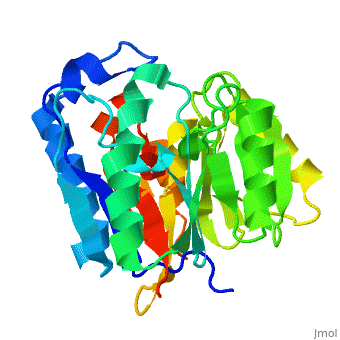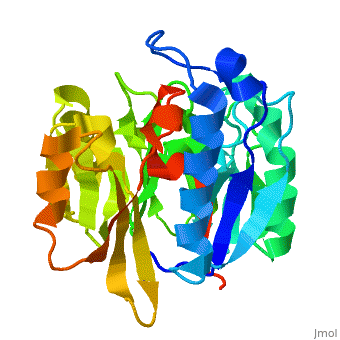Dimethylarginine Dimethylaminohydrolase
From Proteopedia
(Difference between revisions)
| (10 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | <StructureSection load=' | + | <StructureSection load='' size='340' side='right' caption='Bovine dimethylarginine dimethylaminohydrolase (PDB code [[2ci3]])' scene='75/752351/Ddah/1'> |
==Introduction== | ==Introduction== | ||
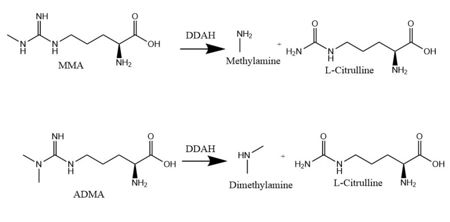
<scene name='75/752351/Ddah/2'>Dimethylarginine Dimethylaminohydrolase</scene> <span class="plainlinks">[http://www.chem.qmul.ac.uk/iubmb/enzyme/EC3/5/3/18.html EC 3.5.3.18]</span> (commonly known as DDAH) is a member of the <span class="plainlinks">[https://en.wikipedia.org/wiki/Hydrolase hydrolase]</span> family of enzymes which use water to break down molecules <ref name="palm">Palm F, Onozato ML, Luo Z, Wilcox CS. Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. American Journal of Physiology. 2007 Dec 1;293(6):3227-3245. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/17933965 17933965]</span> doi:<span class="plainlinks">[http://ajpheart.physiology.org/content/293/6/H3227 10.1152/ajpheart.00998.2007]</span></ref>. Additionally, DDAH is a <span class="plainlinks">[https://en.wikipedia.org/wiki/Nitric_oxide_synthase nitric oxide synthase (NOS)]</span> regulator. It metabolizes free arginine derivatives, namely <span class="plainlinks">[https://en.wikipedia.org/wiki/Asymmetric_dimethylarginine N<sup>Ѡ</sup>,N<sup>Ѡ</sup>-dimethyl-L-arginine (ADMA)]</span> and <span class="plainlinks">[https://en.wikipedia.org/wiki/Methylarginine N<sup>Ѡ</sup>-methyl-L-arginine (MMA)]</span>, which competitively inhibit NOS <ref name="tran">Tran CTL, Leiper JM, Vallance P. The DDAH/ADMA/NOS pathway. Atherosclerosis Supplements. 2003 Dec;4(4):33-40. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/14664901 14664901]</span> doi:<span class="plainlinks">[http://www.sciencedirect.com/science/article/pii/S1567568803000321 10.1016/S1567-5688(03)00032-1]</span></ref>. DDAH converts MMA or ADMA to two products: <span class="plainlinks">[https://en.wikipedia.org/wiki/Citrulline L-citrulline]</span> and an amine <ref name="frey">Frey D, Braun O, Briand C, Vasak M, Grutter MG. Structure of the mammalian NOS regulator dimethylarginine dimethylaminohydrolase: a basis for the design of specific inhibitors. Structure. 2006 May;14(5):901-911. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/16698551]</span> doi:<span class="plainlinks">[http://www.sciencedirect.com/science/article/pii/S0969212606001717 10.1016/j.str.2006.03.006]</span></ref> (Figure 1). DDAH is expressed in the cytosol of cells in humans, mice, rats, sheep, cattle, and bacteria <ref name="palm" />. DDAH activity has been localized mainly to the brain, kidneys, pancreas, and liver in these organisms. Presented in this page is information from DDAH isoform 1 (DDAH-1); however, there are two different isoforms <ref name="frey" />. | <scene name='75/752351/Ddah/2'>Dimethylarginine Dimethylaminohydrolase</scene> <span class="plainlinks">[http://www.chem.qmul.ac.uk/iubmb/enzyme/EC3/5/3/18.html EC 3.5.3.18]</span> (commonly known as DDAH) is a member of the <span class="plainlinks">[https://en.wikipedia.org/wiki/Hydrolase hydrolase]</span> family of enzymes which use water to break down molecules <ref name="palm">Palm F, Onozato ML, Luo Z, Wilcox CS. Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. American Journal of Physiology. 2007 Dec 1;293(6):3227-3245. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/17933965 17933965]</span> doi:<span class="plainlinks">[http://ajpheart.physiology.org/content/293/6/H3227 10.1152/ajpheart.00998.2007]</span></ref>. Additionally, DDAH is a <span class="plainlinks">[https://en.wikipedia.org/wiki/Nitric_oxide_synthase nitric oxide synthase (NOS)]</span> regulator. It metabolizes free arginine derivatives, namely <span class="plainlinks">[https://en.wikipedia.org/wiki/Asymmetric_dimethylarginine N<sup>Ѡ</sup>,N<sup>Ѡ</sup>-dimethyl-L-arginine (ADMA)]</span> and <span class="plainlinks">[https://en.wikipedia.org/wiki/Methylarginine N<sup>Ѡ</sup>-methyl-L-arginine (MMA)]</span>, which competitively inhibit NOS <ref name="tran">Tran CTL, Leiper JM, Vallance P. The DDAH/ADMA/NOS pathway. Atherosclerosis Supplements. 2003 Dec;4(4):33-40. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/14664901 14664901]</span> doi:<span class="plainlinks">[http://www.sciencedirect.com/science/article/pii/S1567568803000321 10.1016/S1567-5688(03)00032-1]</span></ref>. DDAH converts MMA or ADMA to two products: <span class="plainlinks">[https://en.wikipedia.org/wiki/Citrulline L-citrulline]</span> and an amine <ref name="frey">Frey D, Braun O, Briand C, Vasak M, Grutter MG. Structure of the mammalian NOS regulator dimethylarginine dimethylaminohydrolase: a basis for the design of specific inhibitors. Structure. 2006 May;14(5):901-911. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/16698551]</span> doi:<span class="plainlinks">[http://www.sciencedirect.com/science/article/pii/S0969212606001717 10.1016/j.str.2006.03.006]</span></ref> (Figure 1). DDAH is expressed in the cytosol of cells in humans, mice, rats, sheep, cattle, and bacteria <ref name="palm" />. DDAH activity has been localized mainly to the brain, kidneys, pancreas, and liver in these organisms. Presented in this page is information from DDAH isoform 1 (DDAH-1); however, there are two different isoforms <ref name="frey" />. | ||
| - | [[Image:DDAH mechanism.jpg| | + | [[Image:DDAH mechanism.jpg|450px|left|thumb|'''Figure 1.''' The normal DDAH mechanism]] |
| + | {{Clear}} | ||
==Different Isoforms== | ==Different Isoforms== | ||
| Line 17: | Line 18: | ||
====Lid Region Conservation==== | ====Lid Region Conservation==== | ||
The specific residues in the lid region vary between organisms <ref name="frey" /> (Figure 2). Notable in this image is a <span class="plainlinks">[https://en.wikipedia.org/wiki/Conserved_sequence conserved]</span> leucine <scene name='75/752351/Hbond_leu29/9'>(Leu29)</scene> residue in this led that functions to hydrogen bond with the <span class="plainlinks">[https://en.wikipedia.org/wiki/Ligand ligand]</span> bound to the active site in DDAH-1 but not in DDAH-2 <ref name="rasheed" /> (Figure 2). Different <span class="plainlinks">[https://en.wikipedia.org/wiki/Protein_isoform isoforms]</span> from the same species can have differences in lid regions as well <ref name="frey" />. DDAH-2 has a negatively charged lid while DDAH-1 has a positively charged lid <ref name="frey" />. | The specific residues in the lid region vary between organisms <ref name="frey" /> (Figure 2). Notable in this image is a <span class="plainlinks">[https://en.wikipedia.org/wiki/Conserved_sequence conserved]</span> leucine <scene name='75/752351/Hbond_leu29/9'>(Leu29)</scene> residue in this led that functions to hydrogen bond with the <span class="plainlinks">[https://en.wikipedia.org/wiki/Ligand ligand]</span> bound to the active site in DDAH-1 but not in DDAH-2 <ref name="rasheed" /> (Figure 2). Different <span class="plainlinks">[https://en.wikipedia.org/wiki/Protein_isoform isoforms]</span> from the same species can have differences in lid regions as well <ref name="frey" />. DDAH-2 has a negatively charged lid while DDAH-1 has a positively charged lid <ref name="frey" />. | ||
| - | [[Image:WebLogo for Lid Region.png| | + | [[Image:WebLogo for Lid Region.png|450px|left|thumb|'''Figure 2.''' WebLogo for the lid region in DDAH-1 of eleven different organisms.]] |
| - | + | {{Clear}} | |
===Active Site=== | ===Active Site=== | ||
The normal DDAH regulation <span class="plainlinks">[https://en.wikipedia.org/wiki/Reaction_mechanism mechanism]</span> depends on the presence of <scene name='75/752351/Ddah_active_site/4'>Cys249</scene> in the active site that acts as a <span class="plainlinks">[https://en.wikipedia.org/wiki/Nucleophile nucleophile]</span> in the mechanism <ref name="stone">Stone EM, Costello AL, Tierney DL, Fast W. Substrate-assisted cysteine deprotonation in the mechanism of Dimethylargininase (DDAH) from Pseudomonas aeruginosa. Biochemistry. 2006 May 2;45(17):5618-5630. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/16634643 16634643]</span> doi:<span class="plainlinks">[http://pubs.acs.org/doi/abs/10.1021/bi052595m 10.1021/bi052595m]</span></ref> (Figure 3). The Cys249 is used to attack the <span class="plainlinks">[https://en.wikipedia.org/wiki/Guanidine guanidinium]</span> carbon on the substrate that is held in the active site via <scene name='75/752351/Hbond_leu29/6'>hydrogen bonds</scene>. This is followed by collapsing the tetrahedral product to get rid of the <span class="plainlinks">[https://en.wikipedia.org/wiki/Alkylamines alkylamine]</span> leaving group. A <span class="plainlinks">[https://en.wikipedia.org/wiki/Isothiouronium thiouronium]</span> intermediate is then formed with <span class="plainlinks">[https://en.wikipedia.org/wiki/Orbital_hybridisation sp<sup>2</sup> hybridization]</span>. This intermediate is hydrolyzed to form L-citrulline. The <scene name='75/752351/Ddah_active_site_his162/2'>His162</scene> protonates the leaving group in this reaction and generates hydroxide to hydrolyze the intermediate formed in the reaction (Figure 3). L-citrulline leaves the active site when the lid opens. The amines can either leave through the entrance to the active site or through the <scene name='75/752351/Ddah_water_pore/13'>water-filled pore</scene> <ref name="frey" />. Studies suggest that Cys249 is neutral until binding of guanidinium near Cys249 decreases Cys249’s <span class="plainlinks">[https://en.wikipedia.org/wiki/Acid_dissociation_constant pKa]</span> and deprotonates the thiolate to activate the nucleophile <ref name="stone" />. Other studies suggest that the Cys249 and an active site His162 form an <span class="plainlinks">[https://en.wikipedia.org/wiki/Intimate_ion_pair ion pair]</span> to deprotonate the thiolate. Cys249 and His162 can also form a binding site for inhibitors to bind to which stabilizes the thiolate. This is important in regulating NO activity in organisms and designing drugs to inhibit this enzyme <ref name="stone" />. | The normal DDAH regulation <span class="plainlinks">[https://en.wikipedia.org/wiki/Reaction_mechanism mechanism]</span> depends on the presence of <scene name='75/752351/Ddah_active_site/4'>Cys249</scene> in the active site that acts as a <span class="plainlinks">[https://en.wikipedia.org/wiki/Nucleophile nucleophile]</span> in the mechanism <ref name="stone">Stone EM, Costello AL, Tierney DL, Fast W. Substrate-assisted cysteine deprotonation in the mechanism of Dimethylargininase (DDAH) from Pseudomonas aeruginosa. Biochemistry. 2006 May 2;45(17):5618-5630. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/16634643 16634643]</span> doi:<span class="plainlinks">[http://pubs.acs.org/doi/abs/10.1021/bi052595m 10.1021/bi052595m]</span></ref> (Figure 3). The Cys249 is used to attack the <span class="plainlinks">[https://en.wikipedia.org/wiki/Guanidine guanidinium]</span> carbon on the substrate that is held in the active site via <scene name='75/752351/Hbond_leu29/6'>hydrogen bonds</scene>. This is followed by collapsing the tetrahedral product to get rid of the <span class="plainlinks">[https://en.wikipedia.org/wiki/Alkylamines alkylamine]</span> leaving group. A <span class="plainlinks">[https://en.wikipedia.org/wiki/Isothiouronium thiouronium]</span> intermediate is then formed with <span class="plainlinks">[https://en.wikipedia.org/wiki/Orbital_hybridisation sp<sup>2</sup> hybridization]</span>. This intermediate is hydrolyzed to form L-citrulline. The <scene name='75/752351/Ddah_active_site_his162/2'>His162</scene> protonates the leaving group in this reaction and generates hydroxide to hydrolyze the intermediate formed in the reaction (Figure 3). L-citrulline leaves the active site when the lid opens. The amines can either leave through the entrance to the active site or through the <scene name='75/752351/Ddah_water_pore/13'>water-filled pore</scene> <ref name="frey" />. Studies suggest that Cys249 is neutral until binding of guanidinium near Cys249 decreases Cys249’s <span class="plainlinks">[https://en.wikipedia.org/wiki/Acid_dissociation_constant pKa]</span> and deprotonates the thiolate to activate the nucleophile <ref name="stone" />. Other studies suggest that the Cys249 and an active site His162 form an <span class="plainlinks">[https://en.wikipedia.org/wiki/Intimate_ion_pair ion pair]</span> to deprotonate the thiolate. Cys249 and His162 can also form a binding site for inhibitors to bind to which stabilizes the thiolate. This is important in regulating NO activity in organisms and designing drugs to inhibit this enzyme <ref name="stone" />. | ||
| - | [[Image:The Normal DDAH Mechanism.jpg| | + | [[Image:The Normal DDAH Mechanism.jpg|500px|left|thumb|'''Figure 3.''' The normal mechanism of DDAH highlighting important residues involved.]] |
| - | + | {{Clear}} | |
====Channel with Salt Bridge and Water Pore==== | ====Channel with Salt Bridge and Water Pore==== | ||
There is a channel in the center of the protein that is closed by a <scene name='75/752351/Ddah_salt_bridge/6'>salt bridge</scene> connecting Glu77 and Lys174 <ref name="frey" />. This salt bridge constitutes the bottom of the active site. There is a pore containing water on one side of the channel. This pore is <scene name='75/752351/Ddah_water_pore/14'>delineated</scene> by the first β strand of each of the five propeller blades. The water in the channel forms hydrogen bonds to <scene name='75/752351/Ddah_water_pore/15'>His172 and Ser175</scene>. | There is a channel in the center of the protein that is closed by a <scene name='75/752351/Ddah_salt_bridge/6'>salt bridge</scene> connecting Glu77 and Lys174 <ref name="frey" />. This salt bridge constitutes the bottom of the active site. There is a pore containing water on one side of the channel. This pore is <scene name='75/752351/Ddah_water_pore/14'>delineated</scene> by the first β strand of each of the five propeller blades. The water in the channel forms hydrogen bonds to <scene name='75/752351/Ddah_water_pore/15'>His172 and Ser175</scene>. | ||
| Line 29: | Line 30: | ||
Active sites of DDAH from different organisms are similar. Amino acids involved in the chemical mechanism of creating products are also <scene name='69/694225/Evolutionary_conservation/3'>conserved</scene> (Figure 4). | Active sites of DDAH from different organisms are similar. Amino acids involved in the chemical mechanism of creating products are also <scene name='69/694225/Evolutionary_conservation/3'>conserved</scene> (Figure 4). | ||
[[Image:ColorKey ConSurf NoYellow NoGray.gif|400px|right|thumb|'''Figure 4.''' Color key for DDAH conservation]] | [[Image:ColorKey ConSurf NoYellow NoGray.gif|400px|right|thumb|'''Figure 4.''' Color key for DDAH conservation]] | ||
| - | + | {{Clear}} | |
====Zn(II) Bound to the Active Site==== | ====Zn(II) Bound to the Active Site==== | ||
<span class="plainlinks">[https://en.wikipedia.org/wiki/Zinc Zinc (Zn(II))]</span> acts as an <span class="plainlinks">[https://en.wikipedia.org/wiki/Endogeny_(biology) endogenous]</span> inhibitor of DDAH <ref name="frey" /> (Figure 5). The Zn(II)-binding site is located inside the protein’s active site, making it a <span class="plainlinks">[https://en.wikipedia.org/wiki/Competitive_inhibition competitive inhibitor]</span>. When bound, Zn(II) <scene name='69/694225/Closed_lid_zn9/6'>blocks the entrance</scene> of any other substrate. When <span class="plainlinks">[https://en.wikipedia.org/wiki/Crystallization crystallized]</span> with Zn(II) at <scene name='69/694225/Active_site6/2'>pH 6.3</scene>, an open conformation of the lid region has been shown; however, when Zn(II) is bound at <scene name='69/694225/Active_site_9/3'>pH 9.0</scene>, a closed lid has been observed (Figure 5). | <span class="plainlinks">[https://en.wikipedia.org/wiki/Zinc Zinc (Zn(II))]</span> acts as an <span class="plainlinks">[https://en.wikipedia.org/wiki/Endogeny_(biology) endogenous]</span> inhibitor of DDAH <ref name="frey" /> (Figure 5). The Zn(II)-binding site is located inside the protein’s active site, making it a <span class="plainlinks">[https://en.wikipedia.org/wiki/Competitive_inhibition competitive inhibitor]</span>. When bound, Zn(II) <scene name='69/694225/Closed_lid_zn9/6'>blocks the entrance</scene> of any other substrate. When <span class="plainlinks">[https://en.wikipedia.org/wiki/Crystallization crystallized]</span> with Zn(II) at <scene name='69/694225/Active_site6/2'>pH 6.3</scene>, an open conformation of the lid region has been shown; however, when Zn(II) is bound at <scene name='69/694225/Active_site_9/3'>pH 9.0</scene>, a closed lid has been observed (Figure 5). | ||
| - | [[Image:Zn(II) bound at differing pH values.jpg|500 px|center|thumb|'''Figure 5.''' Zn(II) bound to the active site of DDAH at differing pH values. A) Zn(II) bound at pH 9.0 showing the channel of DDAH. B) Zn(II) bound at 9.0 showing the closed conformation lid with Leu29 blocking the active site. C) Zn(II) bound at pH 6.3 showing the channel of DDAH. D) Zn(II) bound at pH 6.3 showing the open lid conformation with Leu29 away from the active site.]] | ||
| + | [[Image:Zn(II) bound at differing pH values.jpg|450px|left|thumb|'''Figure 5.''' Zn(II) bound to the active site of DDAH at differing pH values. A) Zn(II) bound at pH 9.0 showing the channel of DDAH. B) Zn(II) bound at 9.0 showing the closed conformation lid with Leu29 blocking the active site. C) Zn(II) bound at pH 6.3 showing the channel of DDAH. D) Zn(II) bound at pH 6.3 showing the open lid conformation with Leu29 away from the active site.]] | ||
| + | {{Clear}} | ||
=====Important residues in Zinc Binding===== | =====Important residues in Zinc Binding===== | ||
It was found that Cys273, His172, Glu77, Asp78, and Asp 268 all <scene name='69/694225/Active_site6hbonds/3'>play a role</scene> in the binding of Zn(II). <scene name='69/694225/Cys273_zn/2'>Cys273</scene> directly coordinates with the Zn(II) ion in the active site while the other significant residues stabilize the ion via hydrogen bonding interactions with water molecules in the active site. Depending on pH, His172 can change conformation. At pH 9.0, DDAH-1 has been crystalized with <scene name='69/694225/Active_site_9/2'>His172</scene> in both conformations. Both of these conformations use the <span class="plainlinks">[https://en.wikipedia.org/wiki/Imidazole imidazole]</span> group to directly coordinate the Zn(II) ion. Cys273, which is conserved between bovine and humans, is the key active site residue that coordinates Zn(II) <ref name="frey" />. Zinc-cysteine complexes have been found to be important mediators of protein <span class="plainlinks">[https://en.wikipedia.org/wiki/Catalysis catalysis]</span>, regulation, and structure <ref name="pace">Pace NJ, Weerpana E. Zinc-binding cysteines: diverse functions and structural motifs. Biomolecules. 2014 June;4(2):419-434. PMCID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4101490/ 4101490]</span> doi:<span class="plainlinks">[http://www.mdpi.com/2218-273X/4/2/419/htm 10.3390/biom4020419]</span> </ref>. Cys273 and the water molecules stabilize the Zn(II) ion in a tetrahedral environment. The Zn(II) dissociation constant is 4.2 nM which is consistent with the nanomolar concentrations of Zn(II) in the cells, which provides more evidence for the regulatory use of Zn(II) by DDAH <ref name="pace" />. | It was found that Cys273, His172, Glu77, Asp78, and Asp 268 all <scene name='69/694225/Active_site6hbonds/3'>play a role</scene> in the binding of Zn(II). <scene name='69/694225/Cys273_zn/2'>Cys273</scene> directly coordinates with the Zn(II) ion in the active site while the other significant residues stabilize the ion via hydrogen bonding interactions with water molecules in the active site. Depending on pH, His172 can change conformation. At pH 9.0, DDAH-1 has been crystalized with <scene name='69/694225/Active_site_9/2'>His172</scene> in both conformations. Both of these conformations use the <span class="plainlinks">[https://en.wikipedia.org/wiki/Imidazole imidazole]</span> group to directly coordinate the Zn(II) ion. Cys273, which is conserved between bovine and humans, is the key active site residue that coordinates Zn(II) <ref name="frey" />. Zinc-cysteine complexes have been found to be important mediators of protein <span class="plainlinks">[https://en.wikipedia.org/wiki/Catalysis catalysis]</span>, regulation, and structure <ref name="pace">Pace NJ, Weerpana E. Zinc-binding cysteines: diverse functions and structural motifs. Biomolecules. 2014 June;4(2):419-434. PMCID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4101490/ 4101490]</span> doi:<span class="plainlinks">[http://www.mdpi.com/2218-273X/4/2/419/htm 10.3390/biom4020419]</span> </ref>. Cys273 and the water molecules stabilize the Zn(II) ion in a tetrahedral environment. The Zn(II) dissociation constant is 4.2 nM which is consistent with the nanomolar concentrations of Zn(II) in the cells, which provides more evidence for the regulatory use of Zn(II) by DDAH <ref name="pace" />. | ||
| Line 40: | Line 42: | ||
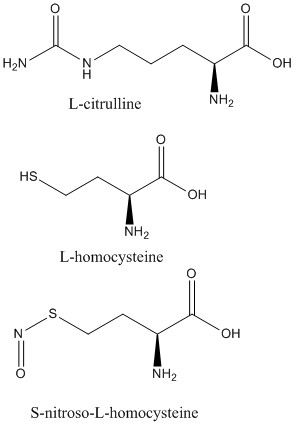
<scene name='75/752351/Ddah_l-homocysteine/3'>L-homocysteine</scene> and <scene name='75/752351/Ddah_with_l-citrulline/5'>L-citrulline</scene> bind in the active site in the same orientation as MMA and ADMA to create the same <span class="plainlinks">[https://en.wikipedia.org/wiki/Intermolecular_force intermolecular bonds]</span> between them and DDAH <ref name="frey" /> (Figure 6). L-citrulline is also a product of DDAH hydrolyzing ADMA and MMA, suggesting DDAH activity creates a <span class="plainlinks">[https://en.wikipedia.org/wiki/Negative_feedback negative feedback]</span> loop on itself (Figure 3). Both molecules enter the active site and cause DDAH to be in its closed lid formation. The α carbon on either molecule creates three <scene name='75/752351/Hbond_leu29/7'>salt bridges</scene> with DDAH: two with the guanidine group of Arg144 and one with the guanidine group on Arg97. Another salt bridge is formed between the ligand and Asp72. The molecules are stabilized in the active site by <scene name='75/752351/Hbond_leu29/4'>four hydrogen bonds</scene>: α carbon-amino group of the ligand to main chain carbonyls of Val267 and Leu29. Hydrogen bonds also form between the side chains of Asp78 and Glu77 with the ureido group of L-citrulline. | <scene name='75/752351/Ddah_l-homocysteine/3'>L-homocysteine</scene> and <scene name='75/752351/Ddah_with_l-citrulline/5'>L-citrulline</scene> bind in the active site in the same orientation as MMA and ADMA to create the same <span class="plainlinks">[https://en.wikipedia.org/wiki/Intermolecular_force intermolecular bonds]</span> between them and DDAH <ref name="frey" /> (Figure 6). L-citrulline is also a product of DDAH hydrolyzing ADMA and MMA, suggesting DDAH activity creates a <span class="plainlinks">[https://en.wikipedia.org/wiki/Negative_feedback negative feedback]</span> loop on itself (Figure 3). Both molecules enter the active site and cause DDAH to be in its closed lid formation. The α carbon on either molecule creates three <scene name='75/752351/Hbond_leu29/7'>salt bridges</scene> with DDAH: two with the guanidine group of Arg144 and one with the guanidine group on Arg97. Another salt bridge is formed between the ligand and Asp72. The molecules are stabilized in the active site by <scene name='75/752351/Hbond_leu29/4'>four hydrogen bonds</scene>: α carbon-amino group of the ligand to main chain carbonyls of Val267 and Leu29. Hydrogen bonds also form between the side chains of Asp78 and Glu77 with the ureido group of L-citrulline. | ||
Like L-homocysteine and L-citrulline, <scene name='75/752351/Ddah_s-nitroso-l-homocysteine/4'>S-nitroso-L-homocysteine</scene> binds and the lid region of DDAH is closed (Figure 6). When DDAH reacts with S-nitroso-L-homocysteine, a covalent product, N-thiosulfximide exist in the active site because of its binding to Cys273. N-thiosulfximide is stabilized by several salt bridges and hydrogen bonds. Arg144 and Arg97 stabilize the α carbon-carbonyl group via salt bridges, and Leu29, Val267, and Asp72 stabilize the α carbon-amino group by forming hydrogen bonds <ref name="frey" />. | Like L-homocysteine and L-citrulline, <scene name='75/752351/Ddah_s-nitroso-l-homocysteine/4'>S-nitroso-L-homocysteine</scene> binds and the lid region of DDAH is closed (Figure 6). When DDAH reacts with S-nitroso-L-homocysteine, a covalent product, N-thiosulfximide exist in the active site because of its binding to Cys273. N-thiosulfximide is stabilized by several salt bridges and hydrogen bonds. Arg144 and Arg97 stabilize the α carbon-carbonyl group via salt bridges, and Leu29, Val267, and Asp72 stabilize the α carbon-amino group by forming hydrogen bonds <ref name="frey" />. | ||
| - | [[Image:L-citrulline, L-homocysteine, and S-nitroso-L-homocysteine.jpg| | + | [[Image:L-citrulline, L-homocysteine, and S-nitroso-L-homocysteine.jpg|450px|left|thumb|'''Figure 6.''' Structures of DDAH inhibitors.]] |
| - | + | {{Clear}} | |
==Clinical Relevance== | ==Clinical Relevance== | ||
DDAH works to hydrolyze MMA and ADMA <ref name="frey" />. Both MMA and ADMA competitively inhibit NO synthesis by inhibiting Nitric Oxide Synthase (NOS). NO is made by NOS creating L-citrulline from <span class="plainlinks">[https://en.wikipedia.org/wiki/Arginine L-arginine]</span> <ref name="frey" />. If DDAH is overexpressed, NOS activity will subsequently increase <ref name="frey" />. ADMA and MMA can <span class="plainlinks">[https://en.wikipedia.org/wiki/Enzyme_inhibitor inhibit]</span> the synthesis of NO by competitively inhibiting all three kinds of NOS (endothelial, neuronal, and inducible) <ref name="frey" />. Underexpression or inhibition of DDAH decreases NOS activity and NO levels will decrease. Because of <span class="plainlinks">[https://en.wikipedia.org/wiki/Nitric_oxide nitric oxide’s (NO)]</span> role in signaling and defense, NO levels in an organism must be regulated to reduce damage to cells <ref name="janssen">Janssen W, Pullamsetti SS, Cooke J, Weissmann N, Guenther A, Schermuly RT. The role of dimethylarginine dimethylaminohydrolase (DDAH) in pulmonary fibrosis. The Journal of Pathology. 2012 Dec 12;229(2):242-249. Epub 2013 Jan. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/23097221 23097221]</span> doi:<span class="plainlinks">[http://onlinelibrary.wiley.com/doi/10.1002/path.4127/references;jsessionid=C34C6C633A21C2ECE14278BBC902AD71.f03t04?globalMessage=0 10.1002/path.4127]</span></ref>. NO is an important signaling and effector molecule in <span class="plainlinks">[https://en.wikipedia.org/wiki/Neurotransmission neurotransmission]</span>, bacterial defense, and regulation of vascular tone <ref name="colasanti">Colasanti M, Suzuki H. The dual personality of NO. ScienceDirect. 2000 Jul 1;21(7):249-252. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/10979862 10979862]</span> doi:<span class="plainlinks">[http://www.sciencedirect.com/science/article/pii/S0165614700014991 10.1016/S0165-6147(00)01499-1]</span></ref>. Because NO is highly toxic, freely diffusible across membranes, and its radical form is fairly reactive, cells must maintain a large control on concentrations by regulating NOS activity and the activity of enzymes such as DDAH that have an indirect effect of the concentration of NO <ref name="rassaf">Rassaf T, Feelisch M, Kelm M. Circulating NO pool: assessment of nitrite and nitroso species in blood and tissues. Free Rad. Biol. Med. 2004 Feb 15;36(4):413-422. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/14975444 14975444]</span> doi:<span class="plainlinks">[http://www.sciencedirect.com/science/article/pii/S0891584903007962 10.1016/j.freeradbiomed.2003.11.011]</span></ref>. An imbalance of NO contributes to several diseases. Low NO levels, potentially caused by low DDAH activity and therefore high MMA and ADMA concentrations, have been associated with diseases such as <span class="plainlinks">[https://en.wikipedia.org/wiki/Uremia uremia]</span>, <span class="plainlinks">[http://www.mayoclinic.org/diseases-conditions/heart-failure/basics/definition/con-20029801 chronic heart failure]</span>, <span class="plainlinks">[https://en.wikipedia.org/wiki/Atherosclerosis atherosclerosis]</span>, and <span class="plainlinks">[https://en.wikipedia.org/wiki/Hyperhomocysteinemia hyperhomocysteinemia]</span> <ref name="tsao">Tsao PS, Cooke JP. Endothelial alterations in hypercholesterolemia: more than simply vasodilator dysfunction. Journal of Cardiovascular Pharmacology. 1998;32(3):48-53. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/9883748 9883748]</span></ref>. High levels of NO have been involved with diseases such as <span class="plainlinks">[https://en.wikipedia.org/wiki/Septic_shock septic shock]</span>, <span class="plainlinks">[http://www.mayoclinic.org/diseases-conditions/migraine-headache/home/ovc-20202432 migraine]</span>, <span class="plainlinks">[https://en.wikipedia.org/wiki/Inflammation inflammation]</span>, and <span class="plainlinks">[https://en.wikipedia.org/wiki/Neurodegeneration neurodegenerative disorders]</span> <ref name="vallance">Vallance P, Leiper J. Blocking NO synthesis: how, where and why? Nat. Rev. Drug Discov. 2002 Dec;1(12):939-950. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/12461516 12461516]</span> doi:<span class="plainlinks">[http://www.nature.com/nrd/journal/v1/n12/full/nrd960.html 10.1038/nrd960]</span></ref>. Because of the effects on NO levels and known inhibitors to DDAH, regulation of DDAH may be an effective way to regulate NO levels, therefore treating these diseases <ref name="frey" />. Additionally, researchers can take advantage of the fact that there are two different isoforms of this enzyme and create drugs that target one isoform over another to control NO levels in specific tissues in the body <ref name="frey" />. | DDAH works to hydrolyze MMA and ADMA <ref name="frey" />. Both MMA and ADMA competitively inhibit NO synthesis by inhibiting Nitric Oxide Synthase (NOS). NO is made by NOS creating L-citrulline from <span class="plainlinks">[https://en.wikipedia.org/wiki/Arginine L-arginine]</span> <ref name="frey" />. If DDAH is overexpressed, NOS activity will subsequently increase <ref name="frey" />. ADMA and MMA can <span class="plainlinks">[https://en.wikipedia.org/wiki/Enzyme_inhibitor inhibit]</span> the synthesis of NO by competitively inhibiting all three kinds of NOS (endothelial, neuronal, and inducible) <ref name="frey" />. Underexpression or inhibition of DDAH decreases NOS activity and NO levels will decrease. Because of <span class="plainlinks">[https://en.wikipedia.org/wiki/Nitric_oxide nitric oxide’s (NO)]</span> role in signaling and defense, NO levels in an organism must be regulated to reduce damage to cells <ref name="janssen">Janssen W, Pullamsetti SS, Cooke J, Weissmann N, Guenther A, Schermuly RT. The role of dimethylarginine dimethylaminohydrolase (DDAH) in pulmonary fibrosis. The Journal of Pathology. 2012 Dec 12;229(2):242-249. Epub 2013 Jan. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/23097221 23097221]</span> doi:<span class="plainlinks">[http://onlinelibrary.wiley.com/doi/10.1002/path.4127/references;jsessionid=C34C6C633A21C2ECE14278BBC902AD71.f03t04?globalMessage=0 10.1002/path.4127]</span></ref>. NO is an important signaling and effector molecule in <span class="plainlinks">[https://en.wikipedia.org/wiki/Neurotransmission neurotransmission]</span>, bacterial defense, and regulation of vascular tone <ref name="colasanti">Colasanti M, Suzuki H. The dual personality of NO. ScienceDirect. 2000 Jul 1;21(7):249-252. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/10979862 10979862]</span> doi:<span class="plainlinks">[http://www.sciencedirect.com/science/article/pii/S0165614700014991 10.1016/S0165-6147(00)01499-1]</span></ref>. Because NO is highly toxic, freely diffusible across membranes, and its radical form is fairly reactive, cells must maintain a large control on concentrations by regulating NOS activity and the activity of enzymes such as DDAH that have an indirect effect of the concentration of NO <ref name="rassaf">Rassaf T, Feelisch M, Kelm M. Circulating NO pool: assessment of nitrite and nitroso species in blood and tissues. Free Rad. Biol. Med. 2004 Feb 15;36(4):413-422. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/14975444 14975444]</span> doi:<span class="plainlinks">[http://www.sciencedirect.com/science/article/pii/S0891584903007962 10.1016/j.freeradbiomed.2003.11.011]</span></ref>. An imbalance of NO contributes to several diseases. Low NO levels, potentially caused by low DDAH activity and therefore high MMA and ADMA concentrations, have been associated with diseases such as <span class="plainlinks">[https://en.wikipedia.org/wiki/Uremia uremia]</span>, <span class="plainlinks">[http://www.mayoclinic.org/diseases-conditions/heart-failure/basics/definition/con-20029801 chronic heart failure]</span>, <span class="plainlinks">[https://en.wikipedia.org/wiki/Atherosclerosis atherosclerosis]</span>, and <span class="plainlinks">[https://en.wikipedia.org/wiki/Hyperhomocysteinemia hyperhomocysteinemia]</span> <ref name="tsao">Tsao PS, Cooke JP. Endothelial alterations in hypercholesterolemia: more than simply vasodilator dysfunction. Journal of Cardiovascular Pharmacology. 1998;32(3):48-53. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/9883748 9883748]</span></ref>. High levels of NO have been involved with diseases such as <span class="plainlinks">[https://en.wikipedia.org/wiki/Septic_shock septic shock]</span>, <span class="plainlinks">[http://www.mayoclinic.org/diseases-conditions/migraine-headache/home/ovc-20202432 migraine]</span>, <span class="plainlinks">[https://en.wikipedia.org/wiki/Inflammation inflammation]</span>, and <span class="plainlinks">[https://en.wikipedia.org/wiki/Neurodegeneration neurodegenerative disorders]</span> <ref name="vallance">Vallance P, Leiper J. Blocking NO synthesis: how, where and why? Nat. Rev. Drug Discov. 2002 Dec;1(12):939-950. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/12461516 12461516]</span> doi:<span class="plainlinks">[http://www.nature.com/nrd/journal/v1/n12/full/nrd960.html 10.1038/nrd960]</span></ref>. Because of the effects on NO levels and known inhibitors to DDAH, regulation of DDAH may be an effective way to regulate NO levels, therefore treating these diseases <ref name="frey" />. Additionally, researchers can take advantage of the fact that there are two different isoforms of this enzyme and create drugs that target one isoform over another to control NO levels in specific tissues in the body <ref name="frey" />. | ||
| - | </StructureSection> | ||
| - | + | == 3D Structures of dimethylarginine dimethylaminohydrolase 1 == | |
| + | |||
| + | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| + | |||
| + | [[3i2e]], [[6szq]], [[7usz]], [[7ut0]] – hDDAH - human <br /> | ||
| + | [[2jai]] – hDDAH + citrulline <br /> | ||
| + | [[2jaj]], [[3p8e]], [[3i4a]] – hDDAH + ornithine derivative<br /> | ||
| + | [[3p8p]] – hDDAH (mutant) + ornithine derivative<br /> | ||
| + | [[6szp]] – hDDAH + guanidine derivative<br /> | ||
| + | [[6dge]], [[7ulu]], [[7ulv]], [[7ulx]] – hDDAH + inhibitor<br /> | ||
| + | [[2ci3]], [[2ci4]], [[2ci6]], [[2ci7]] – bDDAH – bovine <br /> | ||
| + | [[2c6z]] – bDDAH + citrulline <br /> | ||
| + | [[3bpb]] – bDDAH (mutant) + methylthio-citrulline <br /> | ||
| + | [[2ci5]], [[2ci1]] – bDDAH + L-homocysteine derivative<br /> | ||
| + | [[3rhy]] – PaDDAH + chloro-hydroxymethylpyridine – ''Pseudomonas aeruginosa''<br /> | ||
| + | [[1h70]] – PaDDAH (mutant) + citrulline <br /> | ||
== References == | == References == | ||
{{reflist}} | {{reflist}} | ||
| - | + | </StructureSection> | |
== Student Contributors == | == Student Contributors == | ||
*Natalie Van Ochten | *Natalie Van Ochten | ||
*Kaitlyn Enderle | *Kaitlyn Enderle | ||
*Colton Junod | *Colton Junod | ||
| - | |||
| - | == 3D Structures of Dimethylarginine Dimethylaminohydrolase == | ||
| - | <span class="plainlinks">[http://proteopedia.org/wiki/index.php/2c6z 2C6Z]</span> L-citrulline bound to isoform 1 | ||
| - | |||
| - | <span class="plainlinks">[http://proteopedia.org/wiki/index.php/2ci1 2CI1]</span> S-nitroso-L-homocysteine bound to isoform 1 | ||
| - | |||
| - | <span class="plainlinks">[http://proteopedia.org/wiki/index.php/2ci3 2CI3]</span> crystal form 1 | ||
| - | |||
| - | <span class="plainlinks">[http://proteopedia.org/wiki/index.php/2ci4 2CI4]</span> crystal form 2 | ||
| - | |||
| - | <span class="plainlinks">[http://proteopedia.org/wiki/index.php/2ci5 2CI5]</span> L-homocysteine bound to isoform 1 | ||
| - | |||
| - | <span class="plainlinks">[http://proteopedia.org/wiki/index.php/2ci6 2CI6]</span> Zn (II) bound at low pH to isoform 1 | ||
| - | |||
| - | <span class="plainlinks">[http://proteopedia.org/wiki/index.php/2ci7 2CI7]</span> Zn (II) bound at high pH to isoform 1 | ||
Current revision
| |||||||||||
Student Contributors
- Natalie Van Ochten
- Kaitlyn Enderle
- Colton Junod
Proteopedia Page Contributors and Editors (what is this?)
Natalie Van Ochten, Michal Harel, Alexander Berchansky, Kaitlyn Enderle, Colton Junod, Joel L. Sussman