Sandbox Reserved 1069
From Proteopedia
| (5 intermediate revisions not shown.) | |||
| Line 11: | Line 11: | ||
===Electrostatic Interactions=== | ===Electrostatic Interactions=== | ||
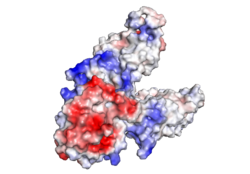
| - | [[Image:ElectrostaticV2.png|250px|right|thumb|Figure 2. Electrostatic Charge Distribution]]<scene name='69/694236/Electrostaticv2/1'>Charge Distribution</scene> along the exterior surface of the protein, shown in Figure 1, is primarily neutral (white) for the TMDs, but transitions to positive (blue) near the location of the <scene name='69/694236/Electrostaticv2salt/2'>salt bridge</scene> and interior side of the cell membrane. This positive section is characteristic of trans-membrane proteins as a means of achieving proper orientation within the cell membrane. <scene name='69/694236/ | + | [[Image:ElectrostaticV2.png|250px|right|thumb|Figure 2. Electrostatic Charge Distribution]]<scene name='69/694236/Electrostaticv2/1'>Charge Distribution</scene> along the exterior surface of the protein, shown in Figure 1, is primarily neutral (white) for the TMDs, but transitions to positive (blue) near the location of the <scene name='69/694236/Electrostaticv2salt/2'>salt bridge</scene> and interior side of the cell membrane. This positive section is characteristic of trans-membrane proteins as a means of achieving proper orientation within the cell membrane. The <scene name='69/694236/Electroabc/1'>binding sites</scene> A, B, and C, as well as the CTDs of both monomers, all possess a high negative charge (red) relative to the other charges present, facilitating the binding and releasing of Zn<sup>2+</sup> ions. The two CTDs are held together by the charge interlock and hydrophobic interactions of the TMDs, despite their electrostatic repulsion. Upon the release of Zn<sup>2+</sup> ions, the CTDs undergo alterations to electronegativity, which enables domain separation. |
===Interlocking Salt Bridge=== | ===Interlocking Salt Bridge=== | ||
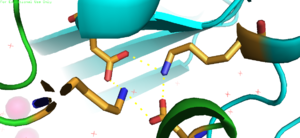
| - | The <scene name='75/756372/Bestsaltbridgetransparent/1'>salt bridge</scene> formation between Lys77 and Asp207 of each domain of YiiP forms an interlocking interaction that acts as the pivot point of the conformational change that drives the function of YiiP as shown in Figure 2. Interlocking interactions are disrupted when Zn<sup>2+</sup> is bound, due to movement of the antiparallel helices, causing a conformational shift in YiiP. The salt bridge also aids in holding the two monomers together, where [[Image:Saltbridge.png| | + | The <scene name='75/756372/Bestsaltbridgetransparent/1'>salt bridge</scene> formation between Lys77 and Asp207 of each domain of YiiP forms an interlocking interaction that acts as the pivot point of the conformational change that drives the function of YiiP as shown in Figure 2. Interlocking interactions are disrupted when Zn<sup>2+</sup> is bound, due to movement of the antiparallel helices, causing a conformational shift in YiiP. The salt bridge also aids in holding the two monomers together, where [[Image:Saltbridge.png|300px|left|thumb|Figure 3. Lys77 and Asp207 Salt Bridges]]<scene name='75/756372/Besthydrophobiczoom1/1'>hydrophobic</scene> residues around the salt bridge further stabilize the two domains in the v-shaped void where the domains connect. This prevents degradation of the protein's interlock via interactions with the environment. |
=== Zn<sup>2+</sup> Binding Sites === | === Zn<sup>2+</sup> Binding Sites === | ||
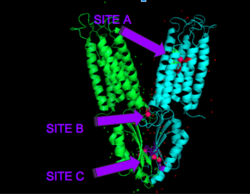
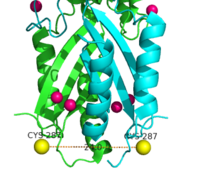
| - | [[Image:activesites.png| | + | [[Image:activesites.png|250px|right|thumb|Figure 4. Binding sites A, B, and C]]Each Yiip monomer contains three Zn<sup>2+</sup> <scene name='69/694236/Bindingsiteswcolor/2'>binding sites</scene>. There is an active site (Site A), and two cytoplasmic binding sites (Site B and C) depicted in Figure 3. It was found that only site A and C are conserved, while the function of Site B is not well defined, though it is believed that it plays a role in subunit dimerization. |
| Line 30: | Line 30: | ||
'''Binding Site C''' | '''Binding Site C''' | ||
| - | <scene name='69/694236/Bsc/1'>Binding site C</scene> has vastly opposite properties from what is seen in binding site A. It is located on a random coil between the two C-terminus domain interfaces. Here, there is a binuclear coordination of Zn<sup>2+</sup> between the Asp285 residue that <scene name='69/694236/Bsc/2'>bridges</scene> the Zn<sup>2+</sup> ions together and the four <scene name='69/694236/Bsc/3'>coordinating</scene> residues (His232, His248, His283, His161). The Asp285 residue does not have outer shell constraints, however, the same cannot be said for the four histidine residues. These constraints consist of hydrogen bonds to the residues surrounding the binding site allowing bidentate bond formation, or hydrogen bonding to a metal in two places.A couple of these potential hydrogen bonds are shown are shown <scene name='69/694236/Bsc/4'> here </scene> Formation of bidentate bonds creates an extensive network of interactions at the CTD interface, which allow for stability and strengthening of the CTD-CTD association. | + | <scene name='69/694236/Bsc/1'>Binding site C</scene> has vastly opposite properties from what is seen in binding site A. It is located on a random coil between the two C-terminus domain interfaces. Here, there is a binuclear coordination of Zn<sup>2+</sup> between the Asp285 residue that <scene name='69/694236/Bsc/2'>bridges</scene> the Zn<sup>2+</sup> ions together and the four <scene name='69/694236/Bsc/3'>coordinating</scene> residues (His232, His248, His283, His161). The Asp285 residue does not have outer shell constraints, however, the same cannot be said for the four histidine residues. These constraints consist of hydrogen bonds to the residues surrounding the binding site allowing bidentate bond formation, or hydrogen bonding to a metal in two places.A couple of these potential hydrogen bonds are shown are shown <scene name='69/694236/Bsc/4'> here </scene>. Formation of bidentate bonds creates an extensive network of interactions at the CTD interface, which allow for stability and strengthening of the CTD-CTD association. |
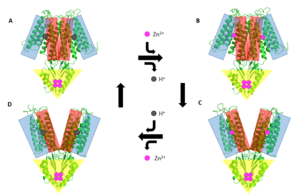
==Mechanism of Transport== | ==Mechanism of Transport== | ||
| Line 56: | Line 56: | ||
[http://proteopedia.org/wiki/index.php/3byp (3byp)] | [http://proteopedia.org/wiki/index.php/3byp (3byp)] | ||
| - | '''Structure of Yiip''' | + | '''Structure of Yiip''' [http://proteopedia.org/wiki/index.php/2qfi (2qfi)] |
| - | [http://proteopedia.org/wiki/index.php/2qfi (2qfi)] | + | |
== References == | == References == | ||
| + | |||
<ref>Fu, Min Lu Dax, and Science21 Sep 2007 : 1746-1748. "Structure of the Zinc Transporter YiiP." Structure of the Zinc Transporter YiiP | Science. Science Magazine, n.d. Web. 24 Feb. 2017.</ref> | <ref>Fu, Min Lu Dax, and Science21 Sep 2007 : 1746-1748. "Structure of the Zinc Transporter YiiP." Structure of the Zinc Transporter YiiP | Science. Science Magazine, n.d. Web. 24 Feb. 2017.</ref> | ||
| - | <ref>"Protein Page: YiiP." Protein Page: YiiP. National Center for Biotechnology Information, n.d. Web. 24 Feb. 2017 | + | <ref>"Protein Page: YiiP." Protein Page: YiiP. National Center for Biotechnology Information, n.d. Web. 24 Feb. 2017</ref> |
| - | <ref>"Laboratory of David Stokes." NYUSOM. NYU School of Medicine, n.d. Web. 24 Feb. 2017 | + | <ref>"Laboratory of David Stokes." NYUSOM. NYU School of Medicine, n.d. Web. 24 Feb. 2017</ref> |
<ref>Plum, Laura M., Lothar Rink, and Hajo Haase. "The Essential Toxin: Impact of Zinc on Human Health." International Journal of Environmental Research and Public Health. Molecular Diversity Preservation International (MDPI), Apr. 2010. Web. 24 Feb. 2017.</ref> | <ref>Plum, Laura M., Lothar Rink, and Hajo Haase. "The Essential Toxin: Impact of Zinc on Human Health." International Journal of Environmental Research and Public Health. Molecular Diversity Preservation International (MDPI), Apr. 2010. Web. 24 Feb. 2017.</ref> | ||
| - | <ref> Fu, Dax. Zinc Transporter YiiP Escherichia Coli (n.d.): n. pag. Brookhaven National Laboratory, Mar. 2010. Web. 21 Apr. 2017 | + | <ref>Fu, Dax. Zinc Transporter YiiP Escherichia Coli (n.d.): n. pag. Brookhaven National Laboratory, Mar. 2010. Web. 21 Apr. 2017</ref> |
| - | <ref> Paulsen, I.T., and Jr. M.H. Saier. "A Novel Family of Ubiquitous Heavy Metal Ion Transport Proteins." SpringerLink. Springer-Verlag, n.d. Web. 21 Apr. 2017 <ref> | + | <ref>Paulsen, I.T., and Jr. M.H. Saier. "A Novel Family of Ubiquitous Heavy Metal Ion Transport Proteins." SpringerLink. Springer-Verlag, n.d. Web. 21 Apr. 2017</ref> |
<references/> | <references/> | ||
Current revision
Introduction
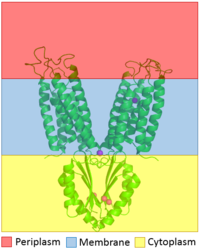
Zinc transporter (TC# 2.A.4.7.1) is an integral membrane protein found in the membrane of Esherichia coli and a member of the cation diffusion facilitator family. Members of this family occur all throughout the biological realm, their primary function being the export of divalent transition metal ions from the cytoplasm to the extracellular space [1]. They work to regulate the amount of divalent metals inside of the cell, which are necessary for different biological functions but can prove to be fatal to the cell in excess amounts. Zinc is essential for the growth and development of cells and zinc levels can affect everything from gene expression to immune response in larger organisms. While YiiP is an integral membrane protein in the cells of Escherichia coli, understanding the mechanism of regulation behind it can help researchers better understand the cation diffusion facilitator equivalents in eukaryotic cells.
| |||||||||||