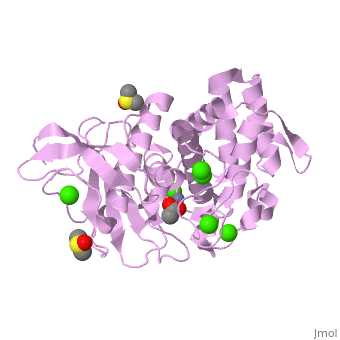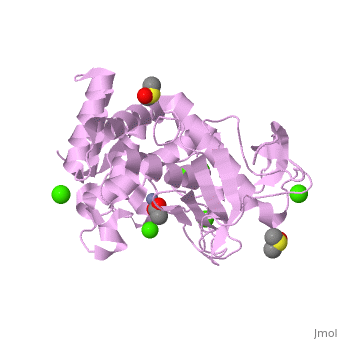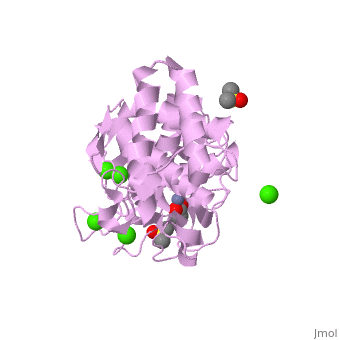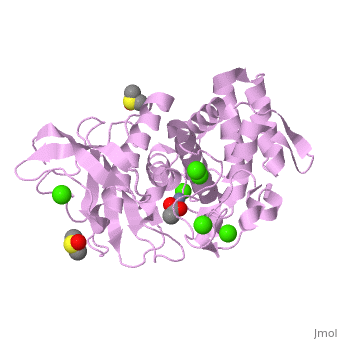Thermolysin
From Proteopedia
(Difference between revisions)
| (3 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | <StructureSection load='2a7g' size=' | + | <StructureSection load='2a7g' size='350' side='right' scene= caption='Thermolysin complex with acetate, DMS, Zn+2 (grey) and Ca+2 (green) ions, [[2a7g]]'> |
== Function == | == Function == | ||
| - | [[Thermolysin]] (TML) is a thermostable metalloproteinase enzyme from ''Bacillus thermoproteolyticus''. It catalyzes the hydrolysis of peptide bonds containing hydrophobic residues. See [[Metalloproteases]] and [[Matrix metalloproteinase]] for discussion. | + | [[Thermolysin]] or '''thermostable neutral proteinase''' (TML) is a thermostable metalloproteinase enzyme from ''Bacillus thermoproteolyticus''. It catalyzes the hydrolysis of peptide bonds containing hydrophobic residues. See [[Metalloproteases]] and [[Matrix metalloproteinase]] for discussion. |
== Structural highlights == | == Structural highlights == | ||
Thermolysin is a well researched [[metalloproteases|metalloprotease]] containing <scene name='User:Ralf_Stephan/Sandbox_2/Zinc/2'>zinc</scene> (click this!) and the amino acids His-Glu-X-His-His as its catalytic center. <scene name='User:Ralf_Stephan/Sandbox_2/Res_yellow/3'>Glu-166, His-142 and -146 are grouped around the zinc atom</scene>, holding it fast, while <scene name='User:Ralf_Stephan/Sandbox_2/Res/1'>Glu-143 holds the polarized water atom. Additionally, Tyr-157 and His-231</scene> stabilize the substrate protein which will be cleaved into two smaller proteins.<ref>Matthews, BW. (1988): ''Structural basis of the action of thermolysin and related zinc peptidases''. In: ''Acc. Chem. Res.'' '''21'''(9); 333–340; http://dx.doi.org/10.1021/ar00153a003</ref><ref>PMID:11935352</ref>. | Thermolysin is a well researched [[metalloproteases|metalloprotease]] containing <scene name='User:Ralf_Stephan/Sandbox_2/Zinc/2'>zinc</scene> (click this!) and the amino acids His-Glu-X-His-His as its catalytic center. <scene name='User:Ralf_Stephan/Sandbox_2/Res_yellow/3'>Glu-166, His-142 and -146 are grouped around the zinc atom</scene>, holding it fast, while <scene name='User:Ralf_Stephan/Sandbox_2/Res/1'>Glu-143 holds the polarized water atom. Additionally, Tyr-157 and His-231</scene> stabilize the substrate protein which will be cleaved into two smaller proteins.<ref>Matthews, BW. (1988): ''Structural basis of the action of thermolysin and related zinc peptidases''. In: ''Acc. Chem. Res.'' '''21'''(9); 333–340; http://dx.doi.org/10.1021/ar00153a003</ref><ref>PMID:11935352</ref>. | ||
| - | </StructureSection> | ||
== 3D Structures of Thermolysin == | == 3D Structures of Thermolysin == | ||
| + | [[Thermolysin 3D structures]] | ||
| - | + | </StructureSection> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | **[[1fjo]], [[1fj3]], [[1fjq]], [[1fjt]], [[1fju]], [[1fjv]], [[1fjw]], [[4tli]], [[5tli]], [[6tli]], [[7tli]], [[8tli]], [[1tli]], [[2tli]], [[3tli]] – TML soaked in organic solvents<br /> | ||
| - | **[[3fv4]], [[3fxp]], [[3flf]], [[3fb0]], [[3fcq]], [[3f28]], [[3f2p]], [[4mtw]], [[4mwp]], [[4mxj]], [[4mzn]], [[4n4e]], [[4n5p]], [[4n66]], [[4oi5]] – TML residues 233-548+inhibitor<br /> | ||
| - | **[[3for]], [[1y3g]], [[1zdp]], [[1z9g]], [[1pes]], [[1pe7]], [[1pe8]], [[1os0]], [[1kto]], [[1ks7]], [[1kro]], [[1kr6]], [[1kkk]], [[1kjo]], [[1kjp]], [[1qf0]], [[1qf1]], [[1qf2]], [[1hyt]], [[1thl]], [[6tmn]], [[7tln]], [[4tln]], [[5tln]], [[1pe5]], [[3ms3]], [[3msa]], [[3msf]], [[3msn]], [[3n21]], [[3nn7]], [[3t73]], [[3t74]], [[3t87]], [[3t8c]], [[3t8d]], [[3t8f]], [[3t8g]], [[3t8h]], [[4h57]], [[4d9w]], [[4d91]], [[5dpe]], [[5dpf]], [[5js3]], [[5jss]], [[5jt9]], [[5jvi]], [[5jxn]], [[5lif]], [[5lvd]], [[5lwd]], [[5m5f]], [[5m69]], [[5mnr]], [[5n2t]], [[5n2x]], [[5n2z]], [[5n31]], [[5n34]], [[5n3v]], [[5n3y]], [[5ma7]], [[5l3u]], [[5l41]], [[5l8p]], [[5m9w]], [[6tmn]], [[7tln]], [[5tln]], [[5tmn]] – TML+inhibitor<br /> | ||
| - | **[[3ssb]] – TML + Impi-α<br /> | ||
| - | **[[3t2h]] – TML + trimethylamine oxide<br /> | ||
| - | **[[3t2i]] – TML + sarcosine<br /> | ||
| - | **[[3t2j]] – TML + betaine<br /> | ||
| - | }} | ||
== References == | == References == | ||
<references/> | <references/> | ||
[[Category: Topic Page]] | [[Category: Topic Page]] | ||
Current revision
| |||||||||||
References
- ↑ Matthews, BW. (1988): Structural basis of the action of thermolysin and related zinc peptidases. In: Acc. Chem. Res. 21(9); 333–340; http://dx.doi.org/10.1021/ar00153a003
- ↑ Pelmenschikov V, Blomberg MR, Siegbahn PE. A theoretical study of the mechanism for peptide hydrolysis by thermolysin. J Biol Inorg Chem. 2002 Mar;7(3):284-98. Epub 2001 Sep 27. PMID:11935352 doi:http://dx.doi.org/10.1007/s007750100295