User:Alec Bertsch/sandbox 1
From Proteopedia
< User:Alec Bertsch(Difference between revisions)
| (43 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
==L5/5S rRNA Complex== | ==L5/5S rRNA Complex== | ||
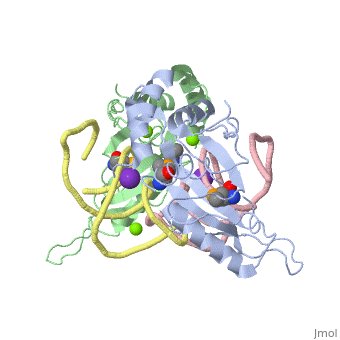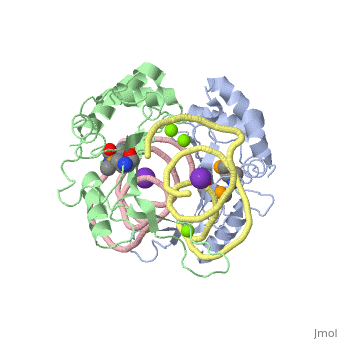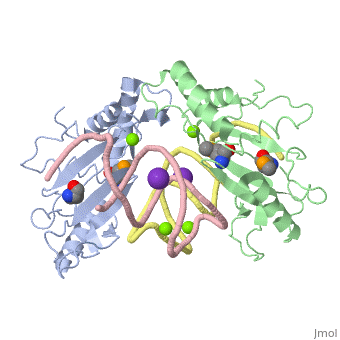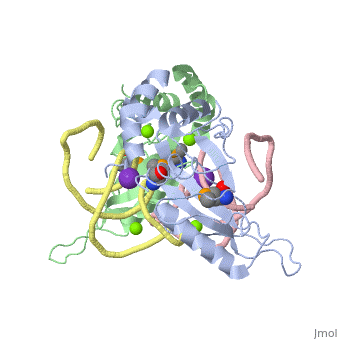
| - | <StructureSection load='1mji' size='340' side='right' caption=' | + | <StructureSection load='1mji' size='340' side='right' caption='3-dimensional view of the L5/5S rRNA complex' scene=''> |
| - | The ribosome is an essential component of all living cells. It is responsible for translation of proteins using messenger RNA (mRNA) and transfer RNA (tRNA). Ribosomes are composed of both proteins and RNA known as ribosomal RNA (rRNA). There are three functional sites found within the ribosome that are essential for translation: the A, P, and E-sites. The A-site is responsible for recruiting the tRNA and the E-site is where the tRNA | + | The ribosome is an essential component of all living cells. It is responsible for translation of proteins using messenger RNA (mRNA) and transfer RNA (tRNA). Ribosomes are composed of both proteins and RNA known as ribosomal RNA (rRNA). There are three functional sites found within the ribosome that are essential for translation: the A, P, and E-sites. The A-site is responsible for recruiting the tRNA and the E-site is where the tRNA exits the ribisome once the protein is removed in the P-site. The site of interest for this sandbox is the P-site, where the protein is removed from the tRNA and added to the growing chain of amino acids. Its primary component is the L5/5S rRNA complex. This complex has been proven to be an essential component to both the structure and the function of the ribosome.<ref>PMID:1370121</ref> |
| - | + | ||
| - | + | ||
== Function == | == Function == | ||
| - | The L5 protein is also responsible for the assembly of the ribosome. In ribosomes that fail to synthesize the L5 protein, the ribosome is unable to connect the large and small units together. | + | The complex is responsible for the stabilization of the ribosomal proteins, mRNA, and tRNA, and is crucial for protein synthesis to occur. The primary function of the L5 protein is to anchor the peptide-linked tRNA to the P-site for the transfer of the peptide to the growing chain of amino acids.<ref>PMID:11497428</ref> |
| - | + | The L5 protein is also responsible for the assembly of the ribosome. In ribosomes that fail to synthesize the L5 protein, the ribosome is unable to connect the large and small sub-units together. Without this protein, the cell would only be able to divide a select few times.<ref>doi:10.1093/nar/gks676</ref> | |
| - | + | The 5S rRNA is responsible for enhancing protein synthesis. This is done by stabilizing the structure of the ribosome through its tight binding to the L5 protein.<ref>PMID:11440169</ref> 5S rRNA has also been noted to be a major component in tumor suppression. This occurs when this rRNA joins with other molecules to form the 5S ribonucleoprotein particle (RNP) which accumlates when ribosome biogenesis is suppressed or blocked.<ref>PMID:27528756</ref> The extent of its role in ribosomal function is not well known, but there have been studies to suggest that the 5S rRNA helps with the binding of the tRNA within the P-site.<ref>PMID:8722002</ref> | |
| - | + | ||
== Structural highlights == | == Structural highlights == | ||
| - | The <scene name='76/769327/Ribosome_l5_protein/2'>L5/5S rRNA complex</scene> makes up the main function and structural component of the large ribosomal sub-unit. | + | The <scene name='76/769327/Ribosome_l5_protein/2'>L5/5S rRNA complex</scene> makes up the main function and structural component of the large ribosomal sub-unit. This image depicts two L5 proteins surrounding two strands of rRNA. |
| - | This | + | <scene name='76/769327/Asp4/1'>Asp4</scene> is one of many residues within the L5 protein that have a crucial role in the binding affinity of 5S rRNA.<ref>PMID:1370121</ref> Mutations on these residues have been shown to drastically decrease they probability of the 5S rRNA binding to the L5 protein. Residues like <scene name='76/769327/Asn40/1'>Asn40</scene> directly influence the binding ability of 5S rRNA. Mutations in these residues result it a less stable interaction between the 5S rRNA and L5 protein. <ref>PMID:1370121</ref> |
| + | The 5S rRNA has many <scene name='76/769327/Conserved_rrna/1'>conserved residues</scene> that contribute to both its structure and function. These residues include C47, U48, C37, C38, A34, and G44. The structural role comes from stacking interactions that occur from the binding of these residues.<ref>PMID:10937990</ref> | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
L5/5S rRNA Complex
| |||||||||||
References
- ↑ Wise JP, Leonard JC, Patierno SR. Clastogenicity of lead chromate particles in hamster and human cells. Mutat Res. 1992 Jan;278(1):69-79. PMID:1370121
- ↑ Meskauskas A, Dinman JD. Ribosomal protein L5 helps anchor peptidyl-tRNA to the P-site in Saccharomyces cerevisiae. RNA. 2001 Aug;7(8):1084-96. PMID:11497428
- ↑ Korepanov AP, Korobeinikova AV, Shestakov SA, Garber MB, Gongadze GM. Protein L5 is crucial for in vivo assembly of the bacterial 50S ribosomal subunit central protuberance. Nucleic Acids Res. 2012 Oct;40(18):9153-9. doi: 10.1093/nar/gks676. Epub 2012 Jul, 20. PMID:22821559 doi:http://dx.doi.org/10.1093/nar/gks676
- ↑ Barciszewska MZ, Szymanski M, Erdmann VA, Barciszewski J. Structure and functions of 5S rRNA. Acta Biochim Pol. 2001;48(1):191-8. PMID:11440169
- ↑ Pelava A, Schneider C, Watkins NJ. The importance of ribosome production, and the 5S RNP-MDM2 pathway, in health and disease. Biochem Soc Trans. 2016 Aug 15;44(4):1086-90. doi: 10.1042/BST20160106. PMID:27528756 doi:http://dx.doi.org/10.1042/BST20160106
- ↑ Bogdanov AA, Dontsova OA, Dokudovskaya SS, Lavrik IN. Structure and function of 5S rRNA in the ribosome. Biochem Cell Biol. 1995 Nov-Dec;73(11-12):869-76. PMID:8722002
- ↑ Wise JP, Leonard JC, Patierno SR. Clastogenicity of lead chromate particles in hamster and human cells. Mutat Res. 1992 Jan;278(1):69-79. PMID:1370121
- ↑ Wise JP, Leonard JC, Patierno SR. Clastogenicity of lead chromate particles in hamster and human cells. Mutat Res. 1992 Jan;278(1):69-79. PMID:1370121
- ↑ Nissen P, Hansen J, Ban N, Moore PB, Steitz TA. The structural basis of ribosome activity in peptide bond synthesis. Science. 2000 Aug 11;289(5481):920-30. PMID:10937990