Vanillyl-alcohol oxidase
From Proteopedia
(Difference between revisions)
| (One intermediate revision not shown.) | |||
| Line 1: | Line 1: | ||
| - | <StructureSection load='' size=' | + | <StructureSection load='' size='350' side='right' caption='Vanillyl-alcohol oxidase containing FAD, [[3fim]]' scene='48/486449/Cv/1' > |
== Function == | == Function == | ||
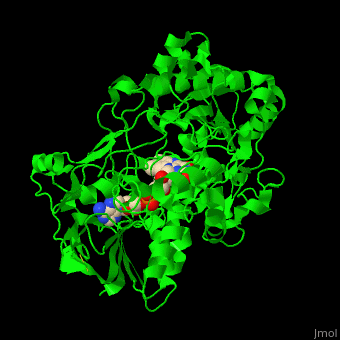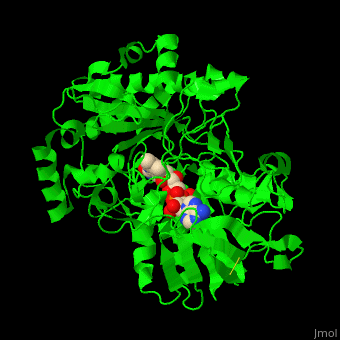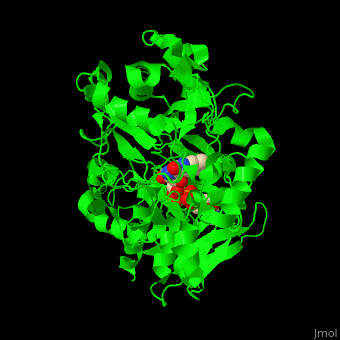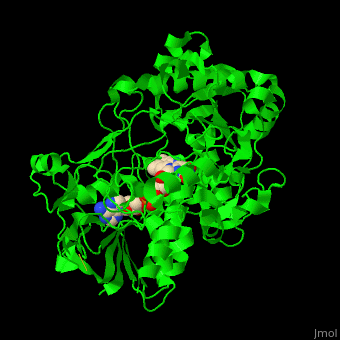
'''Vanillyl-alcohol oxidase''' (VAO) catalyzes the conversion of vanillyl alcohol and molecular oxygen to vaniline and hydrogen peroxide. VAO is part of the 2,4-dichlorobenzoate degradation and uses FAD as a cofactor<ref>PMID:16232469</ref>. | '''Vanillyl-alcohol oxidase''' (VAO) catalyzes the conversion of vanillyl alcohol and molecular oxygen to vaniline and hydrogen peroxide. VAO is part of the 2,4-dichlorobenzoate degradation and uses FAD as a cofactor<ref>PMID:16232469</ref>. | ||
| - | *<scene name='48/486449/Cv/ | + | *<scene name='48/486449/Cv/4'>FAD binding site</scene> in Vanillyl-alcohol oxidase ([[3fim]]). |
== Relevance == | == Relevance == | ||
VAO is a versatile biocatalyst and converts a wide variety of phenolic compounds<ref>PMID:10653736</ref>. | VAO is a versatile biocatalyst and converts a wide variety of phenolic compounds<ref>PMID:10653736</ref>. | ||
Current revision
| |||||||||||
3D structures of vanillyl-alcohol oxidase
Updated on 16-October-2019
References
- ↑ Furukawa H, Wieser M, Morita H, Sugio T, Nagasawa T. Purification and characterization of vanillyl-alcohol oxidase from Byssochlamys fulva V107. J Biosci Bioeng. 1999;87(3):285-90. PMID:16232469
- ↑ Li T, Rosazza JP. Biocatalytic synthesis of vanillin. Appl Environ Microbiol. 2000 Feb;66(2):684-7. PMID:10653736