Vascular Endothelial Growth Factor
From Proteopedia
(Difference between revisions)
| (12 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
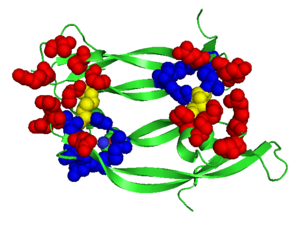
| - | <StructureSection load=' | + | <StructureSection load='.pdb' size='350' side='right' scene='Vascular_Endothelial_Growth_Factor/Vegf-a_opening/1' caption='Structure of Human VEGF-A dimer, [[1vpf]]'> |
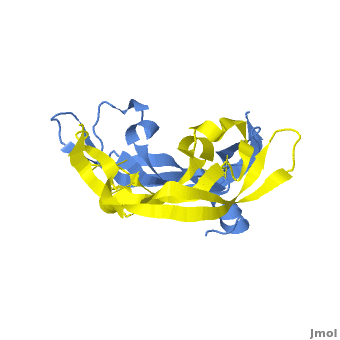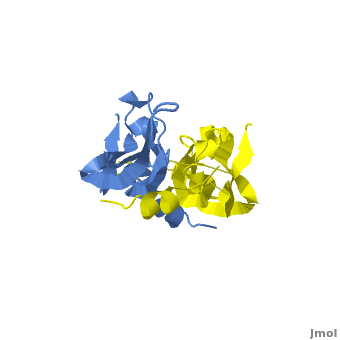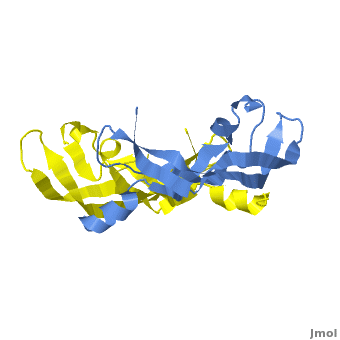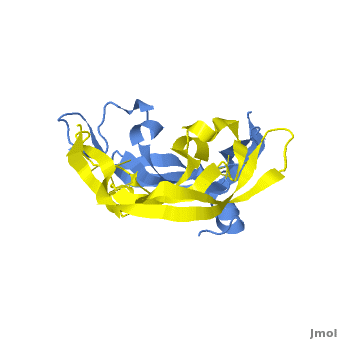
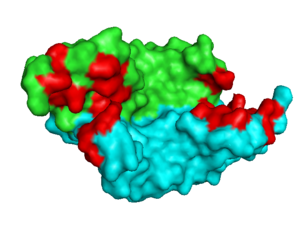
[[Image: VEGF_Opening.png|300px|left|thumb| VEGF-A highlighting 2 monomers and binding sites in red, [[1vpf]]]] | [[Image: VEGF_Opening.png|300px|left|thumb| VEGF-A highlighting 2 monomers and binding sites in red, [[1vpf]]]] | ||
{{Clear}} | {{Clear}} | ||
==Introduction== | ==Introduction== | ||
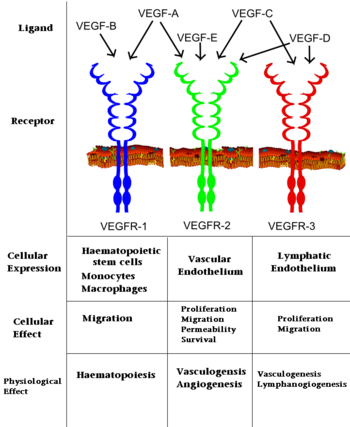
| - | [[Vascular Endothelial Growth Factor]]s (VEGFs) are a class of proteins that regulate vascular development in embryos and angiogenesis in adult mammals after sustaining an injury or notably in [[Cancer|cancerous]] tumors. A number of structural studies have been conducted on VEGF and its receptors (VEGFRs) to better understand the VEGF-VEGFR interaction and how the signal cascade originating from this interaction leads to a number of biological features. VEGF and its receptors have been closely looked at for their potential use as targets for pharmaceutical medicine with some success. The VEGF family contains VEGF-A which mediates increased vascular permeability | + | [[Vascular Endothelial Growth Factor]]s (VEGFs) are a class of proteins that regulate vascular development in embryos and angiogenesis in adult mammals after sustaining an injury or notably in [[Cancer|cancerous]] tumors. A number of structural studies have been conducted on VEGF and its receptors (VEGFRs) to better understand the VEGF-VEGFR interaction and how the signal cascade originating from this interaction leads to a number of biological features. VEGF and its receptors have been closely looked at for their potential use as targets for pharmaceutical medicine with some success. The VEGF family contains: |
| + | *'''VEGF-A''' which mediates increased vascular permeability<ref>PMID:33478167</ref>. | ||
| + | *'''VEGF-B''' which is a growth factor <ref>PMID:19684473</ref>. | ||
| + | *'''VEGF-C''' and '''VEGF-D''' are active in angiogenesis<ref>PMID:9708799</ref>,<ref>PMID:37686121</ref>. | ||
| + | *'''VEGF-E''' is found in viruses<ref>PMID:22507661</ref> | ||
| + | *'''VEGF-F''' found in snake venom<ref>PMID:15542594</ref>.<br /> | ||
| + | For additional information see:<br /> | ||
[[VEGFR]]<br /> | [[VEGFR]]<br /> | ||
| - | [[VEGF IN COMPLEX WITH A NEUTRALIZING ANTIBODY]] | + | [[VEGF IN COMPLEX WITH A NEUTRALIZING ANTIBODY]]<br /> |
| + | [[Autocrine signaling]]<br /> | ||
| + | [[VEGF signaling pathway]]. | ||
<br /> | <br /> | ||
<br /> | <br /> | ||
| Line 40: | Line 48: | ||
VEGF has received significant attention from the pharmaceutical industry in the hopes of correcting impaired vessel function. Such impaired vessel function accompanies many pathologies including atherosclerosis, arthritis, some neurodegenerative diseases such as ALS, and malignant cell growth such as cancerous tumors. <ref> PMID:15488020</ref> In fact, numerous studies highlighted the increased expression of VEGF in cancer cells resulting in tumoral angiogenesis, providing the tumor with the network of blood vessels need to grow and expand. VEGF expression is stimulated by hypoxia, a common characteristic of most newly formed tumors, and genetic mutations such as [[K-ras]] and [[p53]], extremely common mutations present in a majority of cancers. <ref>PMID:20662002</ref> Since angiogenesis in typical adults is infrequent while extremely common in tumors, VEGF serves as a selective therapeutic target for cancer. A number of drugs, like Bevacizumab (a [[monoclonal antibody]] better known as [[Avastin]]) have been developed to interrupt the VEGF-VEGFR connection, with some success. Avastin binds to and inhibits all VEGF-A isoforms and has achieved megablockbuster status by earning over $5 billion in 2009 for Roche. <ref>http://www.foxbusiness.com/story/markets/industries/technology/roche-increased-avastin-sales-efforts-doubles-force/</ref> | VEGF has received significant attention from the pharmaceutical industry in the hopes of correcting impaired vessel function. Such impaired vessel function accompanies many pathologies including atherosclerosis, arthritis, some neurodegenerative diseases such as ALS, and malignant cell growth such as cancerous tumors. <ref> PMID:15488020</ref> In fact, numerous studies highlighted the increased expression of VEGF in cancer cells resulting in tumoral angiogenesis, providing the tumor with the network of blood vessels need to grow and expand. VEGF expression is stimulated by hypoxia, a common characteristic of most newly formed tumors, and genetic mutations such as [[K-ras]] and [[p53]], extremely common mutations present in a majority of cancers. <ref>PMID:20662002</ref> Since angiogenesis in typical adults is infrequent while extremely common in tumors, VEGF serves as a selective therapeutic target for cancer. A number of drugs, like Bevacizumab (a [[monoclonal antibody]] better known as [[Avastin]]) have been developed to interrupt the VEGF-VEGFR connection, with some success. Avastin binds to and inhibits all VEGF-A isoforms and has achieved megablockbuster status by earning over $5 billion in 2009 for Roche. <ref>http://www.foxbusiness.com/story/markets/industries/technology/roche-increased-avastin-sales-efforts-doubles-force/</ref> | ||
<br /> | <br /> | ||
| + | == 3D Structures of VEGF== | ||
| + | [[VEGF 3D Structures]] | ||
| - | </StructureSection> | ||
{{Clear}} | {{Clear}} | ||
| - | == 3D Structures of VEGF== | ||
| - | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| - | {{#tree:id=OrganizedByTopic|openlevels=0| | ||
| - | |||
| - | *VEGF-A | ||
| - | |||
| - | **[[5hhc]], [[5hhd]] – hVEGF-A - human <br /> | ||
| - | **[[1mkg]], [[1mkk]], [[1mjv]] – hVEGF-A (mutant) <br /> | ||
| - | **[[3bdy]], [[2qr0]] - hVEGF-A + hFab light+heavy chain <br /> | ||
| - | **[[4kzn]], [[3qtk]], [[2vpf]], [[1vpf]] - hVEGF-A receptor-binding domain<br /> | ||
| - | **[[2fjg]], [[2fjh]] - hVEGF-A receptor-binding domain+ hFab light+heavy chain <br /> | ||
| - | **[[3p9w]] - hVEGF-A + hFab heavy chain<br /> | ||
| - | **[[5fv1]], [[5fv2]] – hVEGF-A receptor-binding domain + antibody residues 1-108<br /> | ||
| - | **[[4zff]] - hVEGF-A residues 217-315 + hFab light+heavy chain<br /> | ||
| - | **[[1tzh]], [[1tzi]], [[1cz8]] - hVEGF-A + mFab YADS1 light+heavy chain - mouse<br /> | ||
| - | **[[5o4e]] – hVEGF-A (mutant) + IgG γ-1<br /> | ||
| - | **[[4glu]] - hVEGF-A residues 1-102<br /> | ||
| - | **[[1kmx]] - hVEGF-A heparin-binding domain<br /> | ||
| - | **[[1vgh]], [[2vgh]] - hVEGF-A heparin-binding domain - NMR<br /> | ||
| - | **[[1qty]], [[1flt]] – hVEGF-A receptor-binding domain + hFMS-like tyrosine <br /> | ||
| - | **[[1vpp]] – hVEGF-A + receptor blocking peptide <br /> | ||
| - | **[[1bj1]] – hVEGF-A + mFab light+heavy chain fragments <br /> | ||
| - | **[[1kat]] - hVEGF-A receptor binding domain + peptide antagonist - NMR<br /> | ||
| - | **[[3s1b]] - hVEGF-A + Mini-Z<br /> | ||
| - | **[[3s1k]] - hVEGF-A + Z-domain<br /> | ||
| - | **[[4gln]], [[4gls]] - hVEGF-A + D-RFX001<br /> | ||
| - | **[[4deq]] – hVEGF-A /neuropilin-1<br /> | ||
| - | **[[4qaf]] – hVEGF-A + lipocalin-1<br /> | ||
| - | **[[4wpb]] – hVEGF-A + α/βVEGF<br /> | ||
| - | **[[5hhc]], [[5hhd]] – hVEGF-A heterochiral<br /> | ||
| - | **[[5t89]] – hVEGF-A + VEGFR1 <br /> | ||
| - | |||
| - | *VEGF-B | ||
| - | |||
| - | **[[2xac]] – hVEGF-B receptor binding domain+ hVEGFR domain 2<br /> | ||
| - | **[[2vwe]] – hVEGF-B + mFab light+heavy chain fragments <br /> | ||
| - | **[[2c7w]] – hVEGF-B <br /> | ||
| - | **[[3v2a]] – hVEGF-B + VEGF-A<br /> | ||
| - | |||
| - | *VEGF-C | ||
| - | |||
| - | **[[2x1w]], [[2x1x]] – hVEGF-C VEGF homology domain+ VEGFR-2 domains 2 & 3<br /> | ||
| - | **[[4bsk]] - hVEGF-C VEGF homology domain + VEGFR-3 domains 1 & 2<br /> | ||
| - | |||
| - | *VEGF-D | ||
| - | |||
| - | **[[2xv7]] – hVEGF-D VEGF homology domain | ||
| - | |||
| - | *VEGF-E | ||
| - | |||
| - | **[[3v6b]] – VEGF-E + VEGFR 2 – Orf virus<br /> | ||
| - | |||
| - | *VEGF-F | ||
| - | |||
| - | **[[1wq8]], [[1wq9]] - VEGF-F – ''Vipera aspis'' | ||
| - | }} | ||
==Additional Resources== | ==Additional Resources== | ||
For additional information, see: | For additional information, see: | ||
| Line 108: | Line 61: | ||
==References== | ==References== | ||
<references /> | <references /> | ||
| - | + | </StructureSection> | |
| - | + | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, David Canner, Joel L. Sussman, Alexander Berchansky, Wayne Decatur, Jaime Prilusky