|
|
| (One intermediate revision not shown.) |
| Line 1: |
Line 1: |
| - | {{Large structure}}
| + | |
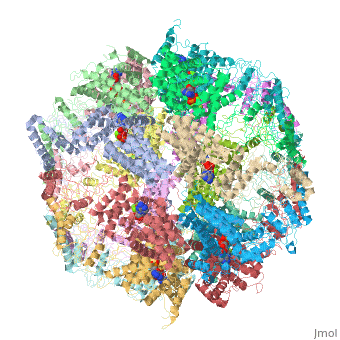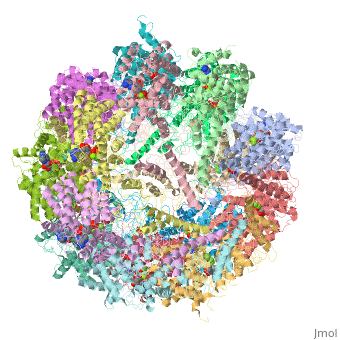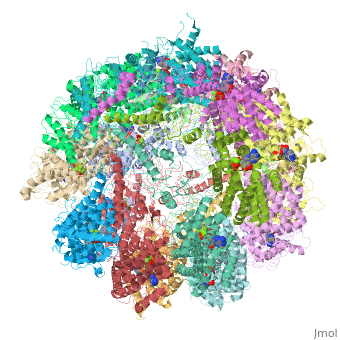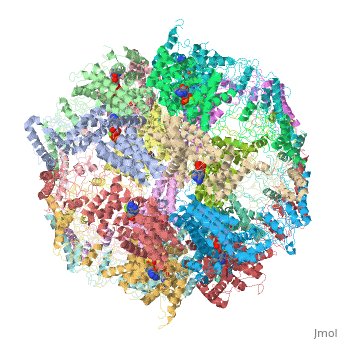
| | ==Molecular architecture of the eukaryotic chaperonin TRiC/CCT derived by a combination of chemical crosslinking and mass-spectrometry, XL-MS== | | ==Molecular architecture of the eukaryotic chaperonin TRiC/CCT derived by a combination of chemical crosslinking and mass-spectrometry, XL-MS== |
| - | <StructureSection load='4v94' size='340' side='right' caption='[[4v94]], [[Resolution|resolution]] 3.80Å' scene=''> | + | <StructureSection load='4v94' size='340' side='right'caption='[[4v94]], [[Resolution|resolution]] 3.80Å' scene=''> |
| | == Structural highlights == | | == Structural highlights == |
| - | <table><tr><td colspan='2'>[[4v94]] is a 32 chain structure with sequence from [http://en.wikipedia.org/wiki/Baker's_yeast Baker's yeast]. This structure supersedes and combines the now removed PDB entries [http://oca.weizmann.ac.il/oca-bin/send-pdb?obs=1&id=4d8q 4d8q] and [http://oca.weizmann.ac.il/oca-bin/send-pdb?obs=1&id=4d8r 4d8r]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=4V94 OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=4V94 FirstGlance]. <br> | + | <table><tr><td colspan='2'>[[4v94]] is a 32 chain structure with sequence from [https://en.wikipedia.org/wiki/Saccharomyces_cerevisiae_S288C Saccharomyces cerevisiae S288C]. This structure supersedes the now removed PDB entries [http://oca.weizmann.ac.il/oca-bin/send-pdb?obs=1&id=4d8q 4d8q] and [http://oca.weizmann.ac.il/oca-bin/send-pdb?obs=1&id=4d8r 4d8r]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=4V94 OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=4V94 FirstGlance]. <br> |
| - | </td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=ADP:ADENOSINE-5-DIPHOSPHATE'>ADP</scene>, <scene name='pdbligand=BEF:BERYLLIUM+TRIFLUORIDE+ION'>BEF</scene>, <scene name='pdbligand=MG:MAGNESIUM+ION'>MG</scene></td></tr> | + | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 3.8Å</td></tr> |
| - | <tr id='related'><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[3p9d|3p9d]], [[3p9e|3p9e]]</td></tr>
| + | <tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=ADP:ADENOSINE-5-DIPHOSPHATE'>ADP</scene>, <scene name='pdbligand=BEF:BERYLLIUM+TRIFLUORIDE+ION'>BEF</scene>, <scene name='pdbligand=MG:MAGNESIUM+ION'>MG</scene></td></tr> |
| - | <tr id='gene'><td class="sblockLbl"><b>[[Gene|Gene:]]</b></td><td class="sblockDat">CCT6, TCP20, TCP6, YD9395.21, YDR188W ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=559292 Baker's yeast]), CCT8, J1374, YJL008C ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=559292 Baker's yeast]), CCT7, J0804, YJL111W ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=559292 Baker's yeast]), CCT5, J1752, TCP5, YJR064W ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=559292 Baker's yeast]), BIN3, CCT2, TCP2, YIL142W ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=559292 Baker's yeast]), ANC2, CCT4, TCP4, YDL143W ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=559292 Baker's yeast]), CCT1, TCP1, YD8142.13, YD8142B.04, YDR212W ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=559292 Baker's yeast]), BIN2, CCT3, J1336, TCP3, YJL014W ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=559292 Baker's yeast])</td></tr> | + | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=4v94 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=4v94 OCA], [https://pdbe.org/4v94 PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=4v94 RCSB], [https://www.ebi.ac.uk/pdbsum/4v94 PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=4v94 ProSAT]</span></td></tr> |
| - | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=4v94 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=4v94 OCA], [http://pdbe.org/4v94 PDBe], [http://www.rcsb.org/pdb/explore.do?structureId=4v94 RCSB], [http://www.ebi.ac.uk/pdbsum/4v94 PDBsum], [http://prosat.h-its.org/prosat/prosatexe?pdbcode=4v94 ProSAT]</span></td></tr> | + | |
| | </table> | | </table> |
| - | {{Large structure}} | |
| | == Function == | | == Function == |
| - | [[http://www.uniprot.org/uniprot/TCPD_YEAST TCPD_YEAST]] Molecular chaperone; assists the folding of proteins upon ATP hydrolysis. Known to play a role, in vitro, in the folding of actin and tubulin. In yeast may play a role in mitotic spindle formation. [[http://www.uniprot.org/uniprot/TCPH_YEAST TCPH_YEAST]] Molecular chaperone; assists the folding of proteins upon ATP hydrolysis. Known to play a role, in vitro, in the folding of actin and tubulin. In yeast may play a role in mitotic spindle formation (By similarity). [[http://www.uniprot.org/uniprot/TCPA_YEAST TCPA_YEAST]] Molecular chaperone; assists the folding of proteins upon ATP hydrolysis. Known to play a role, in vitro, in the folding of actin and tubulin. In yeast may play a role in mitotic spindle formation. [[http://www.uniprot.org/uniprot/TCPQ_YEAST TCPQ_YEAST]] Molecular chaperone; assists the folding of proteins upon ATP hydrolysis. Known to play a role, in vitro, in the folding of actin and tubulin. In yeast may play a role in mitotic spindle formation (By similarity). [[http://www.uniprot.org/uniprot/TCPG_YEAST TCPG_YEAST]] Molecular chaperone; assists the folding of proteins upon ATP hydrolysis. Known to play a role, in vitro, in the folding of actin and tubulin. In yeast may play a role in mitotic spindle formation. [[http://www.uniprot.org/uniprot/TCPZ_YEAST TCPZ_YEAST]] Molecular chaperone; assists the folding of proteins upon ATP hydrolysis. Known to play a role, in vitro, in the folding of actin and tubulin. In yeast may play a role in mitotic spindle formation. [[http://www.uniprot.org/uniprot/TCPE_YEAST TCPE_YEAST]] Molecular chaperone; assists the folding of proteins upon ATP hydrolysis. Known to play a role, in vitro, in the folding of actin and tubulin. In yeast may play a role in mitotic spindle formation. [[http://www.uniprot.org/uniprot/TCPB_YEAST TCPB_YEAST]] Molecular chaperone; assists the folding of proteins upon ATP hydrolysis. Known to play a role, in vitro, in the folding of actin and tubulin. In yeast may play a role in mitotic spindle formation. | + | [https://www.uniprot.org/uniprot/TCPZ_YEAST TCPZ_YEAST] Molecular chaperone; assists the folding of proteins upon ATP hydrolysis. Known to play a role, in vitro, in the folding of actin and tubulin. In yeast may play a role in mitotic spindle formation. |
| - | <div style="background-color:#fffaf0;">
| + | |
| - | == Publication Abstract from PubMed ==
| + | |
| - | The cytosolic chaperonin CCT is a 1-MDa protein-folding machine essential for eukaryotic life. The CCT interactome shows involvement in folding and assembly of a small range of proteins linked to essential cellular processes such as cytoskeleton assembly and cell-cycle regulation. CCT has a classic chaperonin architecture, with two heterogeneous 8-membered rings stacked back-to-back, enclosing a folding cavity. However, the mechanism by which CCT assists folding is distinct from other chaperonins, with no hydrophobic wall lining a potential Anfinsen cage, and a sequential rather than concerted ATP hydrolysis mechanism. We have solved the crystal structure of yeast CCT in complex with actin at 3.8 A resolution, revealing the subunit organisation and the location of discrete patches of co-evolving 'signature residues' that mediate specific interactions between CCT and its substrates. The intrinsic asymmetry is revealed by the structural individuality of the CCT subunits, which display unique configurations, substrate binding properties, ATP-binding heterogeneity and subunit-subunit interactions. The location of the evolutionarily conserved N-terminus of Cct5 on the outside of the barrel, confirmed by mutational studies, is unique to eukaryotic cytosolic chaperonins.
| + | |
| - | | + | |
| - | The crystal structure of yeast CCT reveals intrinsic asymmetry of eukaryotic cytosolic chaperonins.,Dekker C, Roe SM, McCormack EA, Beuron F, Pearl LH, Willison KR EMBO J. 2011 Jun 24. doi: 10.1038/emboj.2011.208. PMID:21701561<ref>PMID:21701561</ref>
| + | |
| | | | |
| - | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br>
| + | ==See Also== |
| - | </div>
| + | *[[Chaperonin 3D structures|Chaperonin 3D structures]] |
| - | <div class="pdbe-citations 4v94" style="background-color:#fffaf0;"></div>
| + | |
| - | == References == | + | |
| - | <references/>
| + | |
| | __TOC__ | | __TOC__ |
| | </StructureSection> | | </StructureSection> |
| - | [[Category: Baker's yeast]] | + | [[Category: Large Structures]] |
| - | [[Category: Aebersold, R]] | + | [[Category: Saccharomyces cerevisiae S288C]] |
| - | [[Category: Bracher, A]] | + | [[Category: Aebersold R]] |
| - | [[Category: Chen, B]] | + | [[Category: Bracher A]] |
| - | [[Category: Chiu, W]] | + | [[Category: Chen B]] |
| - | [[Category: Cong, Y]] | + | [[Category: Chiu W]] |
| - | [[Category: Frydman, J]] | + | [[Category: Cong Y]] |
| - | [[Category: Hartl, F U]] | + | [[Category: Frydman J]] |
| - | [[Category: Holmes, S]] | + | [[Category: Hartl FU]] |
| - | [[Category: Joachimiak, L A]] | + | [[Category: Holmes S]] |
| - | [[Category: Leitner, A]] | + | [[Category: Joachimiak LA]] |
| - | [[Category: Ludtke, S]] | + | [[Category: Leitner A]] |
| - | [[Category: Ma, B]] | + | [[Category: Ludtke S]] |
| - | [[Category: Monkemeyer, L]] | + | [[Category: Ma B]] |
| - | [[Category: Pechmann, S]] | + | [[Category: Monkemeyer L]] |
| - | [[Category: Walzthoeni, T]] | + | [[Category: Pechmann S]] |
| - | [[Category: Atpase]]
| + | [[Category: Walzthoeni T]] |
| - | [[Category: Chaperone]]
| + | |
| - | [[Category: Chaperonin]]
| + | |
| - | [[Category: Cytosol]]
| + | |
| - | [[Category: Molecular chaperone]]
| + | |
| - | [[Category: Nano cage]]
| + | |
| - | [[Category: Protein folding]]
| + | |