Carboxypeptidase A
From Proteopedia
(Difference between revisions)
| (8 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | {{Sandbox_Reserved_Butler_CH462_Sp2015_#}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
| - | =Carboxypeptidase A in ''Bos taurus''= | ||
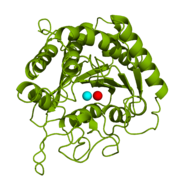
<StructureSection load='1cpx' size='340' side='right' caption='Bovine carboxypeptidase A (CPA)' scene='69/694222/1cpx_default/3'> | <StructureSection load='1cpx' size='340' side='right' caption='Bovine carboxypeptidase A (CPA)' scene='69/694222/1cpx_default/3'> | ||
| Line 13: | Line 11: | ||
===Active Site=== | ===Active Site=== | ||
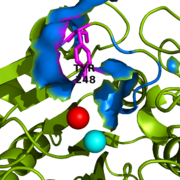
| - | The active site of bovine pancreatic CPA is embedded within a <scene name='69/694222/3cpadeeppocket/1'>deep pocket</scene> (shown in orange); the deep pocket's opening is located on the surface of the protein. Kinetics experiments have indicated that the binding region of the active site is actually capable of extending over five amino acids of the substrate.<ref name="CPA2" /> When no polypeptide substrate is bound in the active site, the pocket is open. However, the pocket is <scene name='69/694222/3cpadeeppocket2/2'>"capped" by a tyrosine residue (Tyr248)</scene> (see section titled "Important Tyr248 Residue") when a substrate or [http://en.wikipedia.org/wiki/Enzyme_inhibitor inhibitor] molecule binds.<ref name="CPA2" /> In this view, the Tyr248 is shown in | + | The active site of bovine pancreatic CPA is embedded within a <scene name='69/694222/3cpadeeppocket/1'>deep pocket</scene> (shown in orange); the deep pocket's opening is located on the surface of the protein. Kinetics experiments have indicated that the binding region of the active site is actually capable of extending over five amino acids of the substrate.<ref name="CPA2" /> When no polypeptide substrate is bound in the active site, the pocket is open. However, the pocket is <scene name='69/694222/3cpadeeppocket2/2'>"capped" by a tyrosine residue (Tyr248)</scene> (see section titled "Important Tyr248 Residue") when a substrate or [http://en.wikipedia.org/wiki/Enzyme_inhibitor inhibitor] molecule binds.<ref name="CPA2" /> In this view, the Tyr248 is shown in magenta. The active site contains two separate subsites, labeled S1' and S1, each of which contain several pertinent residues that serve important roles during the catalyzed hydrolysis reaction. |
=====The Hydrophobic Binding Pocket: S1' Subsite===== | =====The Hydrophobic Binding Pocket: S1' Subsite===== | ||
| - | The <scene name='69/694222/3cpas1primesubsitespacefill/3'>S1' subsite</scene> (spacefill view, subsite in green) contains multiple hydrophobic residues that interact by [http://en.wikipedia.org/wiki/Van_der_Waals_force van der Walls forces] with C-terminal hydrophobic side chains of polypeptide substrates. For this reason, the S1' subsite is referred to as the <scene name='69/694222/3cpas1primesubsitemeshfill/2'>hydrophobic binding pocket</scene> (pseudo-mesh view, subsite in green). It should be | + | The <scene name='69/694222/3cpas1primesubsitespacefill/3'>S1' subsite</scene> (spacefill view, subsite in green) contains multiple hydrophobic residues that interact by [http://en.wikipedia.org/wiki/Van_der_Waals_force van der Walls forces] with C-terminal hydrophobic side chains of polypeptide substrates. For this reason, the S1' subsite is referred to as the <scene name='69/694222/3cpas1primesubsitemeshfill/2'>hydrophobic binding pocket</scene> (pseudo-mesh view, subsite in green). It should be noted that the S1' subsite, despite being named as a hydrophobic pocket, is not another pocket in addition to the deep pocket active site. Rather, it is simply a particular region of the active site. Specifically, the S1' hydrophobic pocket includes the residues <scene name='69/694222/3cpahydrophobicpocketresidues/3'>Ile243, Ile247, Ala250, Gly252, Gly253, Ser254, and Ile255</scene>. The hydrophobic nature of the S1' subsite assists in establishing some degree of [http://en.wikipedia.org/wiki/Chemical_specificity#Enzyme_specificity specificity] for CPA. Because the hydrophobic pocket anchors the polypeptide substrate in the active site, the larger and more hydrophobic the side chain of the C-terminal substrate residue, the stronger the van der Walls interactions between the subsite and the substrate. Therefore, the stability of substrate binding is increased when residues like phenylalanine are present at the C-terminus of the substrate peptide. Essentially, the S1' subsite serves as a recognition site for the C-terminal side chain of the substrate.<ref name="CPA1" /> |
=====S1 Subsite===== | =====S1 Subsite===== | ||
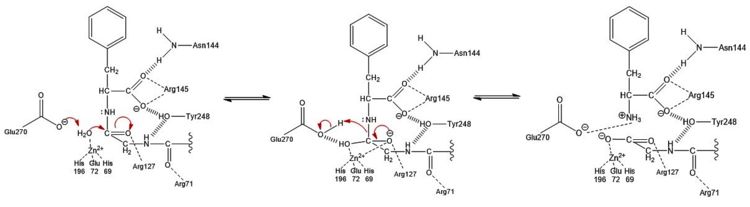
| - | In the same way that the S1' subsite is involved in anchoring the polypeptide substrate in place, the <scene name='69/694222/3cpas1subsitespacefill/3'>S1 subsite</scene> (spacefill view, subsite in magenta) contains several residues that help hold the substrate in the active site, but the S1 subsite also contains the residues that are involved in the catalytic chemical mechanism. In general, the residues of the <scene name='69/694222/3cpas1subsitemeshfill/2'>S1 subsite</scene> (pseudo-mesh view, subsite in magenta) have polar or charged side chains that either allow for hydrogen bonding to stabilize negatively charged intermediates of the hydrolysis reaction or position particular atoms appropriately to allow for chemistry to occur. Three residues (<scene name='69/694222/3cpas1subsiteresidues1/3'>Asn144, Arg145, and Tyr248</scene>) aid in the recognition of the C-terminal residue of a polypeptide substrate.<ref name="CPA1" /> The Asn144 and Tyr248 residues each engage in [http://en.wikipedia.org/wiki/Hydrogen_bond hydrogen bonding] and [http://en.wikipedia.org/wiki/Intermolecular_force ion-dipole interactions] with the carboxyl group at the C-terminus, while Arg145 provides additional stability by participating in [http://www.masterorganicchemistry.com/2010/10/01/how-intermolecular-forces-affect-boiling-points/ ion-ion interactions] with the carboxyl group (see Figure 3 in the section titled "Mechanism of Action"). <scene name='69/694222/3cpas1subsiteresidues2/2'>Arg71</scene> helps stabilize the substrate in the active site by engaging in ion-dipole interactions with the carbonyl oxygen of the penultimate substrate residue (Figure 3). Three residues (<scene name='69/694222/3cpas1subsitezn/3'>His196, Glu72, and His69</scene>) are liganded to a catalytic Zn<sup>2+</sup> ion that is complexed to a water molecule positioned one bond distance away from the C-terminal peptide bond carbonyl carbon (Figure 3). This gives the Zn<sup>2+</sup> ion a tetrahedral binding configuration.<ref name="CPA2" /> <scene name='69/694222/3cpas1subsiteglu270/3'>Glu270</scene> deprotonates this water molecule and acts as a base catalyst in the hydrolysis mechanism (Figure 3). <scene name='69/694222/3cpas1subsitearg127/3'>Arg127</scene> and the positively charged Zn<sup>2+</sup> ion help stabilize the negatively charged intermediate generated in the [http://www.masterorganicchemistry.com/tips/addition-elimination/ addition-elimination] | + | In the same way that the S1' subsite is involved in anchoring the polypeptide substrate in place, the <scene name='69/694222/3cpas1subsitespacefill/3'>S1 subsite</scene> (spacefill view, subsite in magenta) contains several residues that help hold the substrate in the active site, but the S1 subsite also contains the residues that are involved in the catalytic chemical mechanism. In general, the residues of the <scene name='69/694222/3cpas1subsitemeshfill/2'>S1 subsite</scene> (pseudo-mesh view, subsite in magenta) have polar or charged side chains that either allow for hydrogen bonding to stabilize negatively charged intermediates of the hydrolysis reaction or position particular atoms appropriately to allow for chemistry to occur. Three residues (<scene name='69/694222/3cpas1subsiteresidues1/3'>Asn144, Arg145, and Tyr248</scene>) aid in the recognition of the C-terminal residue of a polypeptide substrate.<ref name="CPA1" /> The Asn144 and Tyr248 residues each engage in [http://en.wikipedia.org/wiki/Hydrogen_bond hydrogen bonding] and [http://en.wikipedia.org/wiki/Intermolecular_force ion-dipole interactions] with the carboxyl group at the C-terminus of the peptide substrate, while Arg145 provides additional stability by participating in [http://www.masterorganicchemistry.com/2010/10/01/how-intermolecular-forces-affect-boiling-points/ ion-ion interactions] with the carboxyl group (see Figure 3 in the section titled "Mechanism of Action"). <scene name='69/694222/3cpas1subsiteresidues2/2'>Arg71</scene> helps stabilize the substrate in the active site by engaging in ion-dipole interactions with the carbonyl oxygen of the penultimate substrate residue (Figure 3). Three residues (<scene name='69/694222/3cpas1subsitezn/3'>His196, Glu72, and His69</scene>) are liganded to a catalytic Zn<sup>2+</sup> ion that is complexed to a water molecule positioned one bond distance away from the C-terminal peptide bond carbonyl carbon (Figure 3). This gives the Zn<sup>2+</sup> ion a tetrahedral binding configuration.<ref name="CPA2" /> <scene name='69/694222/3cpas1subsiteglu270/3'>Glu270</scene> deprotonates this water molecule and acts as a base catalyst in the hydrolysis mechanism (Figure 3). <scene name='69/694222/3cpas1subsitearg127/3'>Arg127</scene> and the positively charged Zn<sup>2+</sup> ion help stabilize the negatively charged intermediate generated in the [http://www.masterorganicchemistry.com/tips/addition-elimination/ addition-elimination] hydrolysis reaction (Figure 3).<ref name="CPA2" /> |
=====Putting It All Together===== | =====Putting It All Together===== | ||
| Line 45: | Line 43: | ||
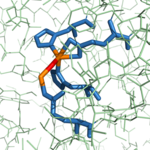
As previously stated, <scene name='69/694222/1cpx_default/3'>CPA</scene> from ''B. taurus'' has the ability to bind two Zn<sup>2+</sup> ions in its active site. The binding of only one Zn<sup>2+</sup> ion is [http://en.wikipedia.org/wiki/Catalysis catalytic], while the binding of a second is [http://en.wikipedia.org/wiki/Reaction_inhibitor inhibitory]. These Zn<sup>2+</sup> ions are connected to each other via a hydroxy-bridge (Figure 4) with a distance of 3.48 [http://en.wikipedia.org/wiki/%C3%85ngstr%C3%B6m Å].<ref name="CPA1" /> The catalytic Zn<sup>2+</sup> ion maintains its tetrahedral binding configuration just as if the inhibitory Zn<sup>2+</sup> ion was not bound. In the CPA structure containing only the catalytic Zn<sup>2+</sup> ion (3CPA), a water molecule complexed to the zinc is able to be deprotonated by <scene name='69/694222/3cpas1subsiteglu270/3'>Glu270</scene>, allowing normal initiation of hydrolysis. Again, this water molecule was not crystallized in the structure of 3CPA, but it is shown in Figure 3. However, when <scene name='69/694222/Glu270wiz/8'>the inhibitory zinc ion</scene> is also present ([http://www.rcsb.org/pdb/explore/explore.do?structureId=1cpx 1CPX]), it occupies the physical space that would normally be occupied by the water molecule. Thus, the inhibitory Zn<sup>2+</sup> ion interacts with the carboxylate group of Glu270. The Glu270 (shown in yellow) now simply stabilizes the second Zn<sup>2+</sup> ion and is unable to perform its usual base catalyst role; the catalytic Zn<sup>2+</sup> ion (shown in cyan) is still being stabilized in place by His69, Glu72, and His196 (shown in orange). | As previously stated, <scene name='69/694222/1cpx_default/3'>CPA</scene> from ''B. taurus'' has the ability to bind two Zn<sup>2+</sup> ions in its active site. The binding of only one Zn<sup>2+</sup> ion is [http://en.wikipedia.org/wiki/Catalysis catalytic], while the binding of a second is [http://en.wikipedia.org/wiki/Reaction_inhibitor inhibitory]. These Zn<sup>2+</sup> ions are connected to each other via a hydroxy-bridge (Figure 4) with a distance of 3.48 [http://en.wikipedia.org/wiki/%C3%85ngstr%C3%B6m Å].<ref name="CPA1" /> The catalytic Zn<sup>2+</sup> ion maintains its tetrahedral binding configuration just as if the inhibitory Zn<sup>2+</sup> ion was not bound. In the CPA structure containing only the catalytic Zn<sup>2+</sup> ion (3CPA), a water molecule complexed to the zinc is able to be deprotonated by <scene name='69/694222/3cpas1subsiteglu270/3'>Glu270</scene>, allowing normal initiation of hydrolysis. Again, this water molecule was not crystallized in the structure of 3CPA, but it is shown in Figure 3. However, when <scene name='69/694222/Glu270wiz/8'>the inhibitory zinc ion</scene> is also present ([http://www.rcsb.org/pdb/explore/explore.do?structureId=1cpx 1CPX]), it occupies the physical space that would normally be occupied by the water molecule. Thus, the inhibitory Zn<sup>2+</sup> ion interacts with the carboxylate group of Glu270. The Glu270 (shown in yellow) now simply stabilizes the second Zn<sup>2+</sup> ion and is unable to perform its usual base catalyst role; the catalytic Zn<sup>2+</sup> ion (shown in cyan) is still being stabilized in place by His69, Glu72, and His196 (shown in orange). | ||
| - | Carboxypeptidase A has been chemically modified and kinetically assayed to determine its Zn<sup>2+</sup> ion | + | Carboxypeptidase A has been chemically modified and kinetically assayed to determine its Zn<sup>2+</sup> ion binding affinities. Literature shows the K<sub>d</sub> value of the catalytic Zn<sup>2+</sup> ion to be two orders of magnitude less than the K<sub>d</sub> value of the inhibitory Zn<sup>2+</sup> ion (K<sub>d</sub> = 2.6x10<sup>-6</sup>M for the catalytic Zn<sup>2+</sup> ion and 5.5x10<sup>-4</sup>M for inhibitory Zn<sup>2+</sup> ion; pH = 8.2). This signifies that the catalytic Zn<sup>2+</sup> ion is approximately one hundred times more likely to bind to CPA compared to the inhibitory Zn<sup>2+</sup> ion.<ref name=“Binding”>Hirose, J., Noji, M., Kidani, Y., Wilkins, R. 1985. Interaction of zinc ions with arsanilazotyrosine-248 carboxypeptidase A.''Biochemistry''. 24(14):3495-3502. [http://pubs.acs.org/doi/abs/10.1021/bi00335a016 DOI:10.1021/bi00335a016]</ref> |
==Other Inhibitors== | ==Other Inhibitors== | ||
| Line 51: | Line 49: | ||
Further detailed studies of anions have indicated that the nature of anion inhibition in the binding site is partly [http://en.wikipedia.org/wiki/Competitive_inhibition competitive].<ref name="CPA1" /> In particular, the sulfate anion (SO<sub>4</sub><sup>2-</sup>) has been of interest to researchers. In a crystallized structure of carboxypeptidase T (PDB code: [http://www.rcsb.org/pdb/explore/explore.do?structureId=1ord 1ORD]), a SO<sub>4</sub><sup>2-</sup> anion was found occupying a portion of a region that corresponds to the amino acid residues Arg127, Asn144, Arg145, and Tyr248 of the S1 subsite of carboxypeptidase A.<ref name="CPA1" /> In this case, it is understood that the SO<sub>4</sub><sup>2-</sup> anion prevents the recognition of the carboxylate group at the C-terminus of the polypeptide substrate. | Further detailed studies of anions have indicated that the nature of anion inhibition in the binding site is partly [http://en.wikipedia.org/wiki/Competitive_inhibition competitive].<ref name="CPA1" /> In particular, the sulfate anion (SO<sub>4</sub><sup>2-</sup>) has been of interest to researchers. In a crystallized structure of carboxypeptidase T (PDB code: [http://www.rcsb.org/pdb/explore/explore.do?structureId=1ord 1ORD]), a SO<sub>4</sub><sup>2-</sup> anion was found occupying a portion of a region that corresponds to the amino acid residues Arg127, Asn144, Arg145, and Tyr248 of the S1 subsite of carboxypeptidase A.<ref name="CPA1" /> In this case, it is understood that the SO<sub>4</sub><sup>2-</sup> anion prevents the recognition of the carboxylate group at the C-terminus of the polypeptide substrate. | ||
| + | |||
| + | ==3D structures of carboxypeptidase A== | ||
| + | |||
| + | See [[Carboxypeptidase]] | ||
</StructureSection> | </StructureSection> | ||
Current revision
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 Bukrinsky JT, Bjerrum MJ, Kadziola A. 1998. Native carboxypeptidase A in a new crystal environment reveals a different conformation of the important tyrosine 248. Biochemistry. 37(47):16555-16564. DOI: 10.1021/bi981678i
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Christianson DW, Lipscomb WN. 1989. Carboxypeptidase A. Acc. Chem. Res. 22:62-69.
- ↑ Suh J, Cho W, Chung S. 1985. Carboxypeptidase A-catalyzed hydrolysis of α-(acylamino)cinnamoyl derivatives of L-β-phenyllactate and L-phenylalaninate: evidence for acyl-enzyme intermediates. J. Am. Chem. Soc. 107:4530-4535. DOI: 10.1021/ja00301a025
- ↑ Hirose, J., Noji, M., Kidani, Y., Wilkins, R. 1985. Interaction of zinc ions with arsanilazotyrosine-248 carboxypeptidase A.Biochemistry. 24(14):3495-3502. DOI:10.1021/bi00335a016
- ↑ Geoghegan, KF, Galdes, A, Martinelli, RA, Holmquist, B, Auld, DS, Vallee, BL. 1983. Cryospectroscopy of intermediates in the mechanism of carboxypeptidase A. Biochem. 22(9):2255-2262. DOI: 10.1021/bi00278a031
- ↑ Kaplan, AP, Bartlett, PA. 1991. Synthesis and evaluation of an inhibitor of carboxypeptidase A with a Ki value in the femtomolar range. Biochem. 30(33):8165-8170. PMID: 1868091
- ↑ Worthington, K., Worthington, V. 1993. Worthington Enzyme Manual: Enzymes and Related Biochemicals. Freehold (NJ): Worthington Biochemical Corporation; [2011; accessed March 28, 2017]. Carboxypeptidase A. http://www.worthington-biochem.com/COA/
- ↑ Pitout, MJ, Nel, W. 1969. The inhibitory effect of ochratoxin a on bovine carboxypeptidase a in vitro. Biochem. Pharma. 18(8):1837-1843. DOI: 0.1016/0006-2952(69)90279-2
- ↑ Normant, E, Martres, MP, Schwartz, JC, Gros, C. 1995. Purification, cDNA cloning, functional expression, and characterization of a 26-kDa endogenous mammalian carboxypeptidase inhibitor. Proc. Natl. Acad. Sci. 92(26):12225-12229. PMCID: PMC40329
Student Contributors
- Thomas Baldwin
- Michael Melbardis
- Clay Schnell
Proteopedia Page Contributors and Editors (what is this?)
Michael Melbardis, Douglas Schnell, Thomas Baldwin, Geoffrey C. Hoops, Michal Harel