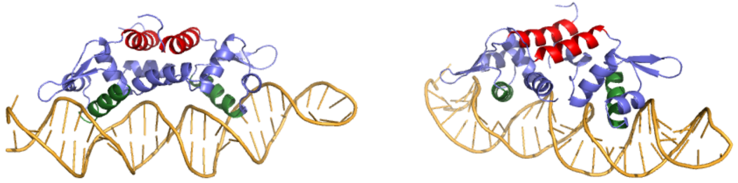We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
CzrA
From Proteopedia
(Difference between revisions)
(New page: =Zinc Dependent Transcriptional Repressor of the Czr operon (CzrA)= <StructureSection load='CzrAwithDNA.pdb' size='340' frame='true' side='right' caption='The dimer Czr A' scene=''> <scene...) |
|||
| (2 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | =Zinc Dependent Transcriptional Repressor of the Czr operon (CzrA)= | ||
<StructureSection load='CzrAwithDNA.pdb' size='340' frame='true' side='right' caption='The dimer Czr A' scene=''> | <StructureSection load='CzrAwithDNA.pdb' size='340' frame='true' side='right' caption='The dimer Czr A' scene=''> | ||
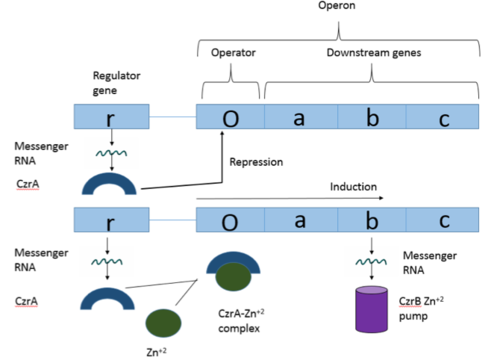
<scene name='69/694220/Czra_with_dna/1'>CzrA</scene> is a transcriptional repressor protein responsible for the regulation of the Czr operon in prokaryotes. The best studied example to date comes from ''Staphylococcus aureus''. | <scene name='69/694220/Czra_with_dna/1'>CzrA</scene> is a transcriptional repressor protein responsible for the regulation of the Czr operon in prokaryotes. The best studied example to date comes from ''Staphylococcus aureus''. | ||
| Line 28: | Line 27: | ||
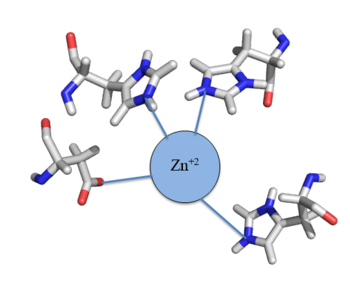
Zn<sup>+2</sup> binding is driven by a large [https://en.wikipedia.org/wiki/Entropy entropic] gain <ref>DOI:10.1021/ja906131b</ref>. Water molecules around the metal ion and Czr A protein are displaced, and gain greater freedom. This gain in entropy allows Zn<sup>+2</sup> to bind to Czr A with reasonable affinity and speed in vivo. The zinc<sup>+2</sup> ion forms a tetrahedral complex with the four residues (Figure 4). Other metal ions that may form a tetrahedral complex will have some affinity for Czr A; however, the metal binding pocket of Czr A has been optimized to bind Zn<sup>+2</sup> with the highest affinity. | Zn<sup>+2</sup> binding is driven by a large [https://en.wikipedia.org/wiki/Entropy entropic] gain <ref>DOI:10.1021/ja906131b</ref>. Water molecules around the metal ion and Czr A protein are displaced, and gain greater freedom. This gain in entropy allows Zn<sup>+2</sup> to bind to Czr A with reasonable affinity and speed in vivo. The zinc<sup>+2</sup> ion forms a tetrahedral complex with the four residues (Figure 4). Other metal ions that may form a tetrahedral complex will have some affinity for Czr A; however, the metal binding pocket of Czr A has been optimized to bind Zn<sup>+2</sup> with the highest affinity. | ||
| + | |||
| + | == 3D Structures of CzrA == | ||
| + | |||
| + | [[CzrA 3D structures]] | ||
</StructureSection> | </StructureSection> | ||
Current revision
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Arunkumar A., Campanello G., Giedroc D. (2009). Solution Structure of a paradigm ArsR family zinc sensor in the DNA-bound state. PNAS 106:43 18177-18182.
- ↑ Chakravorty DK, Wang B, Lee CW, Giedroc DP, Merz KM Jr. Simulations of allosteric motions in the zinc sensor CzrA. J Am Chem Soc. 2012 Feb 22;134(7):3367-76. doi: 10.1021/ja208047b. Epub 2011 Nov , 14. PMID:22007899 doi:http://dx.doi.org/10.1021/ja208047b
- ↑ MacPherson S, Larochelle M, Turcotte B. A fungal family of transcriptional regulators: the zinc cluster proteins. Microbiol Mol Biol Rev. 2006 Sep;70(3):583-604. PMID:16959962 doi:http://dx.doi.org/10.1128/MMBR.00015-06
- ↑ Miller J, McLachlan AD, Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun 4;4(6):1609-1614.
- ↑ Grossoehme NE, Giedroc DP. Energetics of allosteric negative coupling in the zinc sensor S. aureus CzrA. J Am Chem Soc. 2009 Dec 16;131(49):17860-70. doi: 10.1021/ja906131b. PMID:19995076 doi:http://dx.doi.org/10.1021/ja906131b
Student Contributors
- Katelyn Baumer
- Jakob Jozwiakowski
- Catie Liggett