Platelet-derived growth factors and receptors
From Proteopedia
(Difference between revisions)
| (45 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
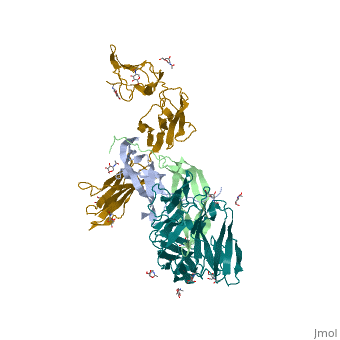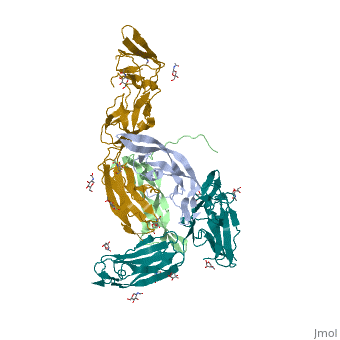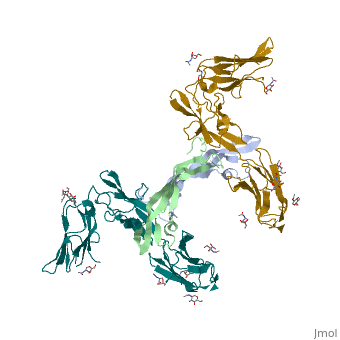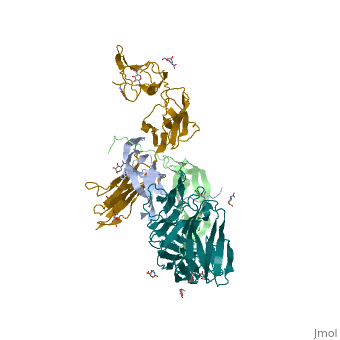
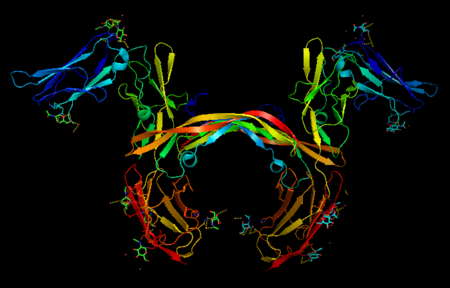
| - | <StructureSection load='3mjg' size='350' side='right' background='none' scene='' caption='Platelet-Derived Growth Factor Receptor (PDB code [[3mjg]])'> | + | <StructureSection load='3mjg' size='350' side='right' background='none' scene='' caption='Platelet-Derived Growth Factor Receptor (brown and turquoise) complex with Platelet-Derived Growth Factor B (grey and green) (PDB code [[3mjg]])'> |
== Overview == | == Overview == | ||
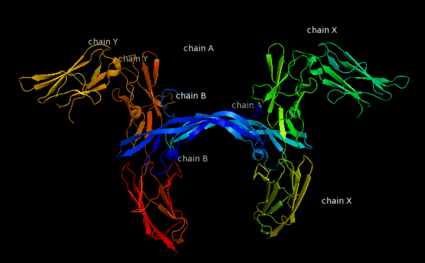
| - | Platelet-derived growth factor receptors, PDGF-R, are cell surface tyrosine kinase receptors for members of the platelet-derived growth factors (PDGFs). PDGF (Chains A and B) is a 172 amino acid sequence consisting of one alpha helix and eight beta-pleated sheets. PDGF function as disulfide-linked dimers. PDGFs have a cysteine-knot-fold growth factor domain of ∼100 amino acids involved in receptor-binding and dimerization. The synthesis and processing of PDGFs is highly regulated. There are two forms of PDGF-R, alpha and beta, which are each encoded by a different gene. Depending on which growth factor binds to the receptor, PDGF-R homodimerizes or heterodimerizes | + | '''Platelet-derived growth factor receptors, PDGF-R''', are cell surface [[Receptor tyrosine kinases|tyrosine kinase receptors]] for members of the '''platelet-derived growth factors (PDGFs)'''. PDGF (Chains A and B) is a 172 amino acid sequence consisting of one alpha helix and eight beta-pleated sheets. PDGF function as disulfide-linked dimers. PDGFs have a cysteine-knot-fold growth factor domain of ∼100 amino acids involved in receptor-binding and dimerization. The synthesis and processing of PDGFs is highly regulated. There are two forms of PDGF-R, alpha and beta, which are each encoded by a different gene. Depending on which growth factor binds to the receptor, PDGF-R homodimerizes or heterodimerizes<ref>PMID:1315403</ref>. The PDGF-R is a 289 amino acid sequence consisting of two alpha helices and twenty-nine beta-pleated sheets. Together these form the PDGF-R complex<ref>PMID:20534510</ref> . |
| - | [[Image:Labeled PDGF-R_r.png|left| | + | [[Image:Labeled PDGF-R_r.png|left|425px]] |
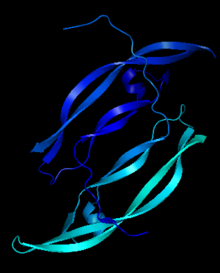
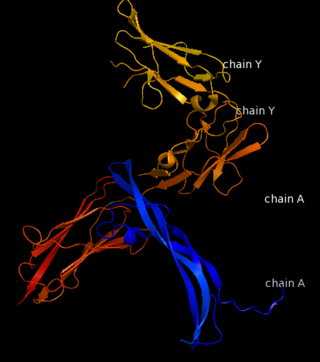
| - | [[Image:Chain_A_and_B_r.png|left| | + | [[Image:Chain_A_and_B_r.png|left|220px]] |
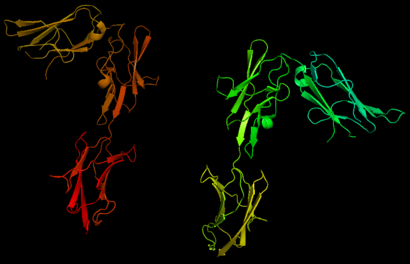
| - | [[Image:Chain_X_and_Y_r.png|right| | + | [[Image:Chain_X_and_Y_r.png|right|410px]] |
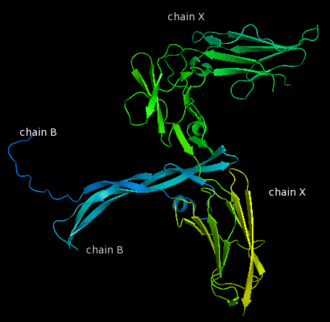
| - | [[Image:Chain B and | + | [[Image:Chain B and X_r.png|left|330px]] |
| - | [[Image:Chain A and | + | [[Image:Chain A and Y_r.png|right|320px]] |
== Mechanism of action == | == Mechanism of action == | ||
| Line 16: | Line 16: | ||
The PDGF family consists of PDGF-A, -B, -C and -D, which form either homo- or heterodimers (PDGF-AA, -AB, -BB, -CC, -DD). These four are inactive in their monomeric forms. However the PDGFs bind to the protein tyrosine kinase receptors PDGF receptor-α and -β. These two receptor isoforms dimerize upon binding of the PDGF dimer, leading to three possible receptor combinations, namely -αα, -ββ and -αβ. The extracellular region of the receptor consists of five immunoglobulin-type domains while the intracellular part is a tyrosine kinase domain. The ligand-binding sites of the receptors are located to the three first immunoglobulin type domains. PDGF-CC specifically interacts with PDGFR-αα and -αβ, but not with -ββ, and thereby resembles PDGF-AB. PDGF-DD binds to PDGFR-ββ with high affinity, and to PDGFR-αβ to a markedly lower extent and is therefore regarded as PDGFR-ββ specific. PDGF-AA binds only to PDGFR-αα, while PDGF-BB is the only PDGF that can bind all three receptor combinations with high affinity. | The PDGF family consists of PDGF-A, -B, -C and -D, which form either homo- or heterodimers (PDGF-AA, -AB, -BB, -CC, -DD). These four are inactive in their monomeric forms. However the PDGFs bind to the protein tyrosine kinase receptors PDGF receptor-α and -β. These two receptor isoforms dimerize upon binding of the PDGF dimer, leading to three possible receptor combinations, namely -αα, -ββ and -αβ. The extracellular region of the receptor consists of five immunoglobulin-type domains while the intracellular part is a tyrosine kinase domain. The ligand-binding sites of the receptors are located to the three first immunoglobulin type domains. PDGF-CC specifically interacts with PDGFR-αα and -αβ, but not with -ββ, and thereby resembles PDGF-AB. PDGF-DD binds to PDGFR-ββ with high affinity, and to PDGFR-αβ to a markedly lower extent and is therefore regarded as PDGFR-ββ specific. PDGF-AA binds only to PDGFR-αα, while PDGF-BB is the only PDGF that can bind all three receptor combinations with high affinity. | ||
| - | Dimerization is a prerequisite for the activation of the kinase. Kinase activation is visualized as tyrosine phosphorylation of the receptor molecules, which occurs between the dimerized receptor molecules (transphosphorylation). In conjunction with dimerization and kinase activation, the receptor molecules undergo conformational changes, which allow a basal kinase activity to phosphorylate a critical tyrosine residue, thereby "unlocking" the kinase, leading to full enzymatic activity directed toward other tyrosine residues in the receptor molecules as well as other substrates for the kinase. This ultimately effects gene expression and cell growth. Expression of both receptors and each of the four PDGFs is under independent control, giving the PDGF/PDGFR system a high flexibility. Different cell types vary greatly in the ratio of PDGF isoforms and PDGFRs expressed. Different external stimuli such as inflammation, embryonic development or differentiation modulate cellular receptor expression allowing binding of some PDGFs but not others. Additionally, some cells display only one of the PDGFR isoforms while other cells express both isoforms, simultaneously or separately. | + | Dimerization is a prerequisite for the activation of the kinase. Kinase activation is visualized as tyrosine phosphorylation of the receptor molecules, which occurs between the dimerized receptor molecules (transphosphorylation). In conjunction with dimerization and kinase activation, the receptor molecules undergo conformational changes, which allow a basal kinase activity to phosphorylate a critical tyrosine residue, thereby "unlocking" the kinase, leading to full enzymatic activity directed toward other tyrosine residues in the receptor molecules as well as other substrates for the kinase. This ultimately effects gene expression and cell growth. Expression of both receptors and each of the four PDGFs is under independent control, giving the PDGF/PDGFR system a high flexibility. Different cell types vary greatly in the ratio of PDGF isoforms and PDGFRs expressed. Different external stimuli such as inflammation, embryonic development or differentiation modulate cellular receptor expression allowing binding of some PDGFs but not others. Additionally, some cells display only one of the PDGFR isoforms while other cells express both isoforms, simultaneously or separately<ref>PMID:2543106</ref> . |
Tyrosine phosphorylation sites in growth factor receptors serve two major purposes: to control the state of activity of the kinase and to create binding sites for downstream signal transduction molecules, which in many cases also are substrates for the kinase. The second part of the tyrosine kinase domain in the PDGFβ receptor is phosphorylated at Tyr-857, and mutant receptors carrying phenylalanine at this position have reduced kinase activity. Tyr-857 has therefore been assigned a role in positive regulation of kinase activity. | Tyrosine phosphorylation sites in growth factor receptors serve two major purposes: to control the state of activity of the kinase and to create binding sites for downstream signal transduction molecules, which in many cases also are substrates for the kinase. The second part of the tyrosine kinase domain in the PDGFβ receptor is phosphorylated at Tyr-857, and mutant receptors carrying phenylalanine at this position have reduced kinase activity. Tyr-857 has therefore been assigned a role in positive regulation of kinase activity. | ||
| Line 24: | Line 24: | ||
== Functionality == | == Functionality == | ||
| - | Platelet-derived growth factor subunits -A and -B are prototypic growth factors which have critical functions in development: regulating cell propagation, cellular differentiation, cell growth, development and many diseases including cancer | + | Platelet-derived growth factor subunits -A and -B are prototypic growth factors which have critical functions in development: regulating cell propagation, cellular differentiation, cell growth, development and many diseases including cancer<ref name="Williams">PMID:2538922</ref>. PDGFs are the major components signaling mitotic cell division in connective tissues and smooth muscle cells. They also critically regulate embryonic development. PDGFα signaling targets such organs as the lung, intestine, skin, kidneys, skeleton and neuroprotective tissues. PDGFβ signaling targets hematopoiesis and blood vessel formation. While, PDGF signaling is imperative during embryonic development and early childhood, it is detrimental to adults. Some PDGF signaling in adults has lead to cancers, atherosclerosis, pulmonary fibrosis, and restenosis. |
| - | + | ||
== Pharmaceutical Ideas == | == Pharmaceutical Ideas == | ||
| Line 31: | Line 30: | ||
Pharmaceutical research has been targeting inhibiting PDGF-PDGFR signaling as a potential for anti-cancer treatments. Strategies of blocking signaling at the cellular level include neutralizing antibiodies for PDGF ligands and receptors, aptamers which are oligonucleic acid or peptide molecules that bind to a specific target molecule, N-terminal processing-deficient PDGFs, and soluble receptors without the kinase domain. | Pharmaceutical research has been targeting inhibiting PDGF-PDGFR signaling as a potential for anti-cancer treatments. Strategies of blocking signaling at the cellular level include neutralizing antibiodies for PDGF ligands and receptors, aptamers which are oligonucleic acid or peptide molecules that bind to a specific target molecule, N-terminal processing-deficient PDGFs, and soluble receptors without the kinase domain. | ||
| + | See also [[PDGFR inhibitors]]. | ||
== Quiz == | == Quiz == | ||
| - | |||
<quiz display=simple> | <quiz display=simple> | ||
| - | { | + | {What kind of receptor is PDGF-R?} |
+A. Cell surface tyrosine kinase receptors | +A. Cell surface tyrosine kinase receptors | ||
-B. Cell surface adenine kinase receptors | -B. Cell surface adenine kinase receptors | ||
| Line 42: | Line 41: | ||
-D. Intracellular receptors | -D. Intracellular receptors | ||
| - | { | + | {The monomeric forms of PDGF and PDGF-R are fully functional.} |
-A. TRUE. | -A. TRUE. | ||
+B. FALSE. | +B. FALSE. | ||
| - | { | + | {The dimerization of the subunits, either in the hetero- or homodimerization forms signals for cellular growth.} |
+A. TRUE. | +A. TRUE. | ||
-B. FALSE. | -B. FALSE. | ||
| - | { | + | {PDGFs are functionally important when? (may be more than one answer)} |
+A. In embryonic development | +A. In embryonic development | ||
-B. In making new neurons. | -B. In making new neurons. | ||
| Line 56: | Line 55: | ||
-D. They are always important during the life cycle, especially as adults. | -D. They are always important during the life cycle, especially as adults. | ||
| - | { | + | {PDGF and PDGF-R are responsible for which of the following? (may be more than one correct answer)} |
-A. signaling cells to lyse. | -A. signaling cells to lyse. | ||
+B. regulating cell propagation, cellular differentiation, cell growth. | +B. regulating cell propagation, cellular differentiation, cell growth. | ||
| Line 62: | Line 61: | ||
-D. Only propagate cell growth in smooth muscle cells. | -D. Only propagate cell growth in smooth muscle cells. | ||
| - | { | + | {PDGF is composed of how many amino acids?} |
-A. 192. | -A. 192. | ||
-B. 272. | -B. 272. | ||
| Line 68: | Line 67: | ||
+D. 172. | +D. 172. | ||
| - | { | + | {PDGF-R is composed of how many amino acids?} |
_A. 172. | _A. 172. | ||
-B. 298. | -B. 298. | ||
| Line 74: | Line 73: | ||
-D. 350. | -D. 350. | ||
| - | { | + | {The four units making up the PDGF-R complex are completely different proteins } |
-A. TRUE. | -A. TRUE. | ||
+B. FALSE. | +B. FALSE. | ||
| - | { | + | {When the receptors undergo a conformational change what occurs? (may be more than one answer).} |
-A. The molecule bends. | -A. The molecule bends. | ||
-B. Kinase activity hydrolyzes a critical tyrosine residue, thereby "unlocking" the kinase, leading to full enzymatic activity directed toward other tyrosine residues in the receptor molecules as well as other substrates for the kinase.. | -B. Kinase activity hydrolyzes a critical tyrosine residue, thereby "unlocking" the kinase, leading to full enzymatic activity directed toward other tyrosine residues in the receptor molecules as well as other substrates for the kinase.. | ||
| Line 84: | Line 83: | ||
- Hydrolase activity phosphorylates a critical tyrosine residue, thereby "unlocking" the kinase, leading to full enzymatic activity directed toward other tyrosine residues in the receptor molecules as well as other substrates for the kinase. | - Hydrolase activity phosphorylates a critical tyrosine residue, thereby "unlocking" the kinase, leading to full enzymatic activity directed toward other tyrosine residues in the receptor molecules as well as other substrates for the kinase. | ||
| - | { | + | {The extracellular region of the receptor contains______ whereas the intracellular region of the receptor contains_________.} |
-A. 4 hemoglobin type domains; tyrosine kinase domain. | -A. 4 hemoglobin type domains; tyrosine kinase domain. | ||
+B. 5 hemoglobin type domains; tyrosine kinase domain. | +B. 5 hemoglobin type domains; tyrosine kinase domain. | ||
| Line 90: | Line 89: | ||
-D. 3 hemoglobin type domains; cytosine kinase domain. | -D. 3 hemoglobin type domains; cytosine kinase domain. | ||
| - | { | + | {The PDGF protein contains ________ alpha helix/helices and _______ beta-pleated sheet(s).} |
+A. 1; 8. | +A. 1; 8. | ||
-B. 2, 8. | -B. 2, 8. | ||
| Line 96: | Line 95: | ||
-D. 1; 28. | -D. 1; 28. | ||
| - | { | + | {The extracellular region of the receptor contains______ whereas the intracellular region of the receptor contains_________.} |
-A. 2;19. | -A. 2;19. | ||
-B. 1;29. | -B. 1;29. | ||
| Line 104: | Line 103: | ||
</StructureSection> | </StructureSection> | ||
| - | == Sources == | ||
| + | ==3D structures of platelet-derived growth factor receptor== | ||
| + | |||
| + | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| + | {{#tree:id=OrganizedByTopic|openlevels=0| | ||
| + | *Platelet-derived growth factor | ||
| - | < | + | **[[3mjk]] – hPDGF subunit A – human<br /> |
| + | **[[4qci]] , [[6t9e]]– hPDGF subunit B + antibody <br /> | ||
| + | **[[1pdg]] – hPDGF BB <br /> | ||
| + | **[[4hqu]], [[4hqx]] – hPDGF BB + DNA<br /> | ||
| - | + | *Platelet-derived growth factor receptor | |
| - | < | + | **[[5k5x]] – hPDGF-R-α kinase domain 550-696, 769-973<br /> |
| + | **[[6a32]] – hPDGF-R-α kinase domain (mutant)<br /> | ||
| + | **[[5grn]] – hPDGF-R-α kinase domain + pyridine derivative <br /> | ||
| + | **[[6jok]], [[6jol]] – hPDGF-R-a residues 550-696, 769-973 + cancer drug<br /> | ||
| + | **[[6joi]], [[6joj]] – hPDGF-R-a residues 550-696, 769-973 (mutant) + cancer drug<br /> | ||
| + | **[[7ram]] – hPDGF-R-a 1-524 + HCMV glycoproteins - CryoEM<br /> | ||
| + | **[[7lbf]] – hPDGF-R-a 1-524 + HCMV glycoproteins + antibody - CryoEM<br /> | ||
| + | **[[3mjg]] – hPDGF-R-β residues 33-314 + PDGF subunit B<br /> | ||
| + | **[[2l6w]] – hPDGF-R-β residues 526-563 - NMR<br /> | ||
| + | }} | ||
| + | ==References== | ||
| - | < | + | <references/> |
| - | + | [[Category:Pages with quizzes]] | |
Current revision
| |||||||||||
3D structures of platelet-derived growth factor receptor
Updated on 05-January-2025
References
- ↑ Heldin CH, Ostman A, Eriksson A, Siegbahn A, Claesson-Welsh L, Westermark B. Platelet-derived growth factor: isoform-specific signalling via heterodimeric or homodimeric receptor complexes. Kidney Int. 1992 Mar;41(3):571-4. PMID:1315403
- ↑ Shim AH, Liu H, Focia PJ, Chen X, Lin PC, He X. Structures of a platelet-derived growth factor/propeptide complex and a platelet-derived growth factor/receptor complex. Proc Natl Acad Sci U S A. 2010 Jun 22;107(25):11307-12. Epub 2010 Jun 2. PMID:20534510
- ↑ Heldin CH, Westermark B. Platelet-derived growth factor: three isoforms and two receptor types. Trends Genet. 1989 Apr;5(4):108-11. PMID:2543106
- ↑ Williams LT. Signal transduction by the platelet-derived growth factor receptor. Science. 1989 Mar 24;243(4898):1564-70. PMID:2538922
Proteopedia Page Contributors and Editors (what is this?)
Joel L. Sussman, Lisa Tice, Michal Harel, Angel Herraez, Jaime Prilusky, Alexander Berchansky