Sandbox Reserved 1329
From Proteopedia
| (5 intermediate revisions not shown.) | |||
| Line 2: | Line 2: | ||
==Human Glucose Transporter GLUT3/SLC2A3== | ==Human Glucose Transporter GLUT3/SLC2A3== | ||
<StructureSection load='5c65' size='340' side='right' caption='Caption for this structure' scene=''> | <StructureSection load='5c65' size='340' side='right' caption='Caption for this structure' scene=''> | ||
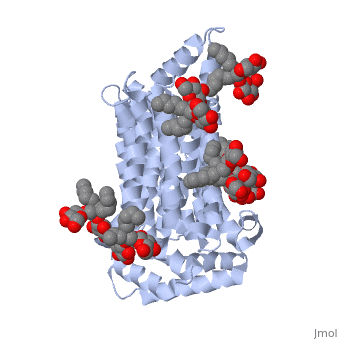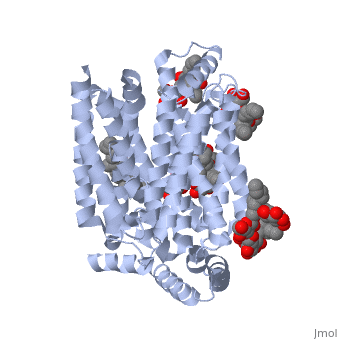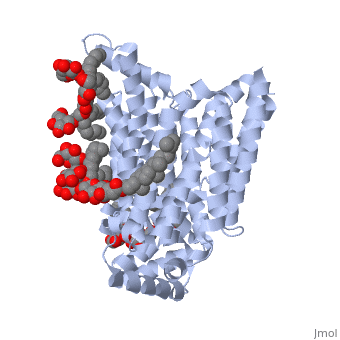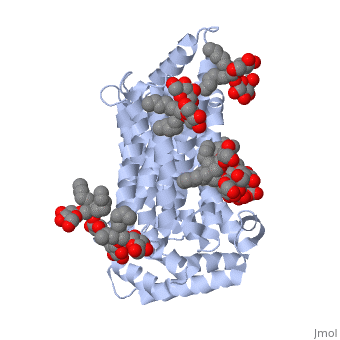
| - | Protein 5c65 is part of the protein class GLUT. The GLUT | + | Protein 5c65 is part of the protein class GLUT. The GLUT protein class facilitates the transport of glucose and hexoses over the plasma membrane is highly evolutionarily conserved across phyla. It is important to note that this protein is an isoform of GLUT 1, a protein which mediates equilibrative glucose transport. Specifically, the GLUT3 protein is mostly expressed in neurons, where it is believed to be the primary glucose transporter. In certain cases, it is expressed to facilitate glucose transport in the placenta. This protein was isolated from human liver cDNA libraries. |
== Function == | == Function == | ||
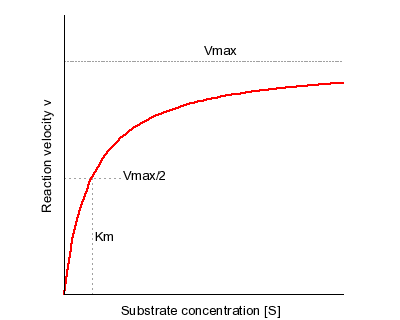
The primary biological function of this protein is for glucose transmembrane transport, or the transport of glucose molecules over the plasma membrane. Using the Michaelis-Mentin Kinetics Model, the GLUT3 mediated transport of glucose had a higher affinity during the outward substrate binding site. Secondly, the transport occurs more efficiently and quickly when the substrate is present on the trans site of the membrane. | The primary biological function of this protein is for glucose transmembrane transport, or the transport of glucose molecules over the plasma membrane. Using the Michaelis-Mentin Kinetics Model, the GLUT3 mediated transport of glucose had a higher affinity during the outward substrate binding site. Secondly, the transport occurs more efficiently and quickly when the substrate is present on the trans site of the membrane. | ||
| + | |||
| + | [[Image:image-1.png]] | ||
== Disease == | == Disease == | ||
| - | Current research has made insights in to the structure of GLUT3, allowing for investigations into variable expression resulting in association with disease processes. Certain GLUT proteins, such as GLUT1 and GLUT3, has increased expression in cancer cells. Analyzing the variation in expression patterns within this protein has been used as a diagnostic tool, and in certain cases it can be used as an imaging tool in oncology. Lastly, there are currently investigations into the applications of GLUT as a drug delivery mechanism due to its binding and conformation properties. | + | Current research has made insights in to the structure of GLUT3, allowing for investigations into variable expression resulting in association with disease processes. Certain GLUT proteins, such as GLUT1 and GLUT3, has increased expression in cancer cells. The most common and well described change of GLUT expression is in cancer cells that switch their metabolism primarily to glycolysis, which is far less energy-efficient and requires far more substrate. This rapid metabolic change is often referred to as the Warburg effect. Analyzing the variation in expression patterns within this protein has been used as a diagnostic tool, and in certain cases it can be used as an imaging tool in oncology. Lastly, there are currently investigations into the applications of GLUT as a drug delivery mechanism due to its binding and conformation properties. |
== Relevance == | == Relevance == | ||
| Line 16: | Line 18: | ||
The protein contains <scene name='77/777649/Helices/1'>12 membrane-spanning alpha helices</scene> and has no known post-translational modifications. The first 6 transmembrane helices are in a pseudo symmetrical configuration relative to the last 6 helices. Helices 1, 2, 4, 5, 7, 8, 10, and 11 form an inner bundle that is stabilized by the outer helices 3, 6, 9, and 12. The GLUT3 protein is comprised of ~500 amino acid residues. It has a single site for N-Linked glycosylation, a central cytoplasmic linker domain, and exhibit topologies with their N and C termini, which are both positioned in the cytoplasm. | The protein contains <scene name='77/777649/Helices/1'>12 membrane-spanning alpha helices</scene> and has no known post-translational modifications. The first 6 transmembrane helices are in a pseudo symmetrical configuration relative to the last 6 helices. Helices 1, 2, 4, 5, 7, 8, 10, and 11 form an inner bundle that is stabilized by the outer helices 3, 6, 9, and 12. The GLUT3 protein is comprised of ~500 amino acid residues. It has a single site for N-Linked glycosylation, a central cytoplasmic linker domain, and exhibit topologies with their N and C termini, which are both positioned in the cytoplasm. | ||
| - | The protein also contains nine <scene name='77/777649/Ligands_37x/1'>37X ligands</scene>. The molecule itself is octyl glucose neopentyl glycol. | + | The protein also contains nine <scene name='77/777649/Ligands_37x/1'>37X ligands</scene>. The molecule itself is octyl glucose neopentyl glycol, which is part of a class of surfactants called glucose-neopentyl glycol (GNG) amphiphiles. GNG amphiphiles are very useful for solubilization and stabilization of membranes |
| - | The protein has another kind of ligand, <scene name='77/777649/Ligands_y01/1'>Y01</scene>. This molecule is cholesterol hemisuccinate. | + | The protein has another kind of ligand, <scene name='77/777649/Ligands_y01/1'>Y01</scene>. This molecule is cholesterol hemisuccinate, which is a membrane stabilizer. This is important for the function of GLUT3 as it transports glucose across the plasma membranes. |
</StructureSection> | </StructureSection> | ||
| Line 24: | Line 26: | ||
== References == | == References == | ||
Carruthers, A., DeZutter, J., Ganguly, A., & Devaskar, S. U. (2009, October). Will the original glucose transporter isoform please stand up! Retrieved February 27, 2018, from https://www.ncbi.nlm.nih.gov/pubmed/19690067 | Carruthers, A., DeZutter, J., Ganguly, A., & Devaskar, S. U. (2009, October). Will the original glucose transporter isoform please stand up! Retrieved February 27, 2018, from https://www.ncbi.nlm.nih.gov/pubmed/19690067 | ||
| + | |||
| + | Chae, P. S., Rana, R. R., Gotfryd, K., Rasmussen, S. G., Kruse, A. C., Cho, K. H., . . . Gellman, S. H. (2013, March 21). Glucose-Neopentyl Glycol (GNG) Amphiphiles for Membrane Protein Solubilization, Stabilization and Crystallization. Retrieved February 27, 2018, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3578972/ | ||
Cheeseman, C., & Long, W. (2015). Structure of, and functional insight into the GLUT family of membrane transporters. Cell Health and Cytoskeleton, 167. doi:10.2147/chc.s60484 | Cheeseman, C., & Long, W. (2015). Structure of, and functional insight into the GLUT family of membrane transporters. Cell Health and Cytoskeleton, 167. doi:10.2147/chc.s60484 | ||
| + | |||
| + | Ding, W. X., Qi, X. R., Li, P., Maitani, Y., & Nagai, T. (2005, August 26). Cholesteryl hemisuccinate as a membrane stabilizer in dipalmitoylphosphatidylcholine liposomes containing saikosaponin-d. Retrieved February 27, 2018, from https://www.ncbi.nlm.nih.gov/pubmed/15978754 | ||
Mueckler, M., & Thorens, B. (2013). Retrieved February 27, 2018, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4104978/ | Mueckler, M., & Thorens, B. (2013). Retrieved February 27, 2018, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4104978/ | ||
Potaman, V. N. (1970, January 01). DNA: Alternative Conformations and Biology. Retrieved February 27, 2018, from https://www.ncbi.nlm.nih.gov/books/NBK6545/ | Potaman, V. N. (1970, January 01). DNA: Alternative Conformations and Biology. Retrieved February 27, 2018, from https://www.ncbi.nlm.nih.gov/books/NBK6545/ | ||
Current revision
| This Sandbox is Reserved from January through July 31, 2018 for use in the course HLSC322: Principles of Genetics and Genomics taught by Genevieve Houston-Ludlam at the University of Maryland, College Park, USA. This reservation includes Sandbox Reserved 1311 through Sandbox Reserved 1430. |
To get started:
More help: Help:Editing |
Human Glucose Transporter GLUT3/SLC2A3
| |||||||||||
References
Carruthers, A., DeZutter, J., Ganguly, A., & Devaskar, S. U. (2009, October). Will the original glucose transporter isoform please stand up! Retrieved February 27, 2018, from https://www.ncbi.nlm.nih.gov/pubmed/19690067
Chae, P. S., Rana, R. R., Gotfryd, K., Rasmussen, S. G., Kruse, A. C., Cho, K. H., . . . Gellman, S. H. (2013, March 21). Glucose-Neopentyl Glycol (GNG) Amphiphiles for Membrane Protein Solubilization, Stabilization and Crystallization. Retrieved February 27, 2018, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3578972/
Cheeseman, C., & Long, W. (2015). Structure of, and functional insight into the GLUT family of membrane transporters. Cell Health and Cytoskeleton, 167. doi:10.2147/chc.s60484
Ding, W. X., Qi, X. R., Li, P., Maitani, Y., & Nagai, T. (2005, August 26). Cholesteryl hemisuccinate as a membrane stabilizer in dipalmitoylphosphatidylcholine liposomes containing saikosaponin-d. Retrieved February 27, 2018, from https://www.ncbi.nlm.nih.gov/pubmed/15978754
Mueckler, M., & Thorens, B. (2013). Retrieved February 27, 2018, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4104978/
Potaman, V. N. (1970, January 01). DNA: Alternative Conformations and Biology. Retrieved February 27, 2018, from https://www.ncbi.nlm.nih.gov/books/NBK6545/