|
|
| (14 intermediate revisions not shown.) |
| Line 1: |
Line 1: |
| - | [[Image:2gk1.gif|left|200px]] | |
| | | | |
| - | {{Structure
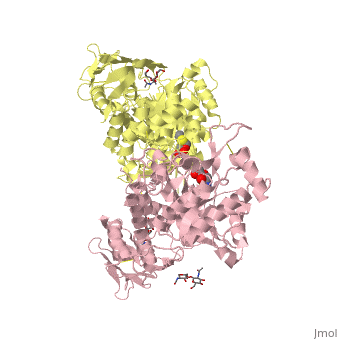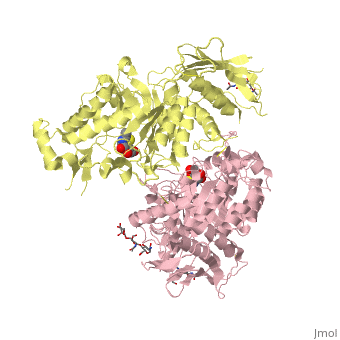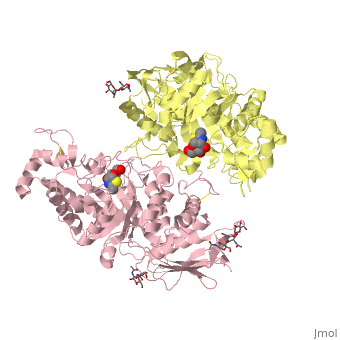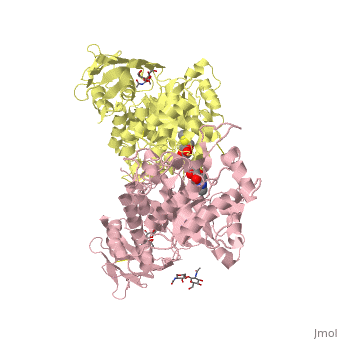
| + | ==X-ray crystal structure of NGT-bound HexA== |
| - | |PDB= 2gk1 |SIZE=350|CAPTION= <scene name='initialview01'>2gk1</scene>, resolution 3.25Å
| + | <StructureSection load='2gk1' size='340' side='right'caption='[[2gk1]], [[Resolution|resolution]] 3.25Å' scene=''> |
| - | |SITE=
| + | == Structural highlights == |
| - | |LIGAND= <scene name='pdbligand=BMA:BETA-D-MANNOSE'>BMA</scene>, <scene name='pdbligand=NAG:N-ACETYL-D-GLUCOSAMINE'>NAG</scene>, <scene name='pdbligand=NGT:3AR,5R,6S,7R,7AR-5-HYDROXYMETHYL-2-METHYL-5,6,7,7A-TETRAHYDRO-3AH-PYRANO[3,2-D]THIAZOLE-6,7-DIOL'>NGT</scene> | + | <table><tr><td colspan='2'>[[2gk1]] is a 20 chain structure with sequence from [https://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2GK1 OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=2GK1 FirstGlance]. <br> |
| - | |ACTIVITY= <span class='plainlinks'>[http://en.wikipedia.org/wiki/Beta-N-acetylhexosaminidase Beta-N-acetylhexosaminidase], with EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=3.2.1.52 3.2.1.52] </span>
| + | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 3.25Å</td></tr> |
| - | |GENE=
| + | <tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=BMA:BETA-D-MANNOSE'>BMA</scene>, <scene name='pdbligand=NAG:N-ACETYL-D-GLUCOSAMINE'>NAG</scene>, <scene name='pdbligand=NGT:3AR,5R,6S,7R,7AR-5-HYDROXYMETHYL-2-METHYL-5,6,7,7A-TETRAHYDRO-3AH-PYRANO[3,2-D]THIAZOLE-6,7-DIOL'>NGT</scene></td></tr> |
| - | |DOMAIN=
| + | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=2gk1 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2gk1 OCA], [https://pdbe.org/2gk1 PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=2gk1 RCSB], [https://www.ebi.ac.uk/pdbsum/2gk1 PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=2gk1 ProSAT]</span></td></tr> |
| - | |RELATEDENTRY=[[2gjx|2GJX]]
| + | </table> |
| - | |RESOURCES=<span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=2gk1 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2gk1 OCA], [http://www.ebi.ac.uk/pdbsum/2gk1 PDBsum], [http://www.rcsb.org/pdb/explore.do?structureId=2gk1 RCSB]</span>
| + | == Disease == |
| - | }}
| + | [https://www.uniprot.org/uniprot/HEXA_HUMAN HEXA_HUMAN] Defects in HEXA are the cause of GM2-gangliosidosis type 1 (GM2G1) [MIM:[https://omim.org/entry/272800 272800]; also known as Tay-Sachs disease. GM2-gangliosidosis is an autosomal recessive lysosomal storage disease marked by the accumulation of GM2 gangliosides in the neuronal cells. GM2G1 is characterized by GM2 gangliosides accumulation in the absence of HEXA activity, leading to neurodegeneration and, in the infantile form, death in early childhood. GM2G1 has an increased incidence among Ashkenazi Jews and French Canadians in eastern Quebec. It exists in several forms: infantile (most common and most severe), juvenile and adult (late onset).<ref>PMID:2970528</ref> <ref>PMID:2522679</ref> <ref>PMID:2144098</ref> <ref>PMID:1837283</ref> <ref>PMID:1532289</ref> <ref>PMID:1302612</ref> <ref>PMID:1301189</ref> <ref>PMID:1301190</ref> <ref>PMID:8490625</ref> <ref>PMID:8445615</ref> <ref>PMID:7951261</ref> <ref>PMID:7837766</ref> <ref>PMID:7717398</ref> <ref>PMID:8581357</ref> <ref>PMID:7898712</ref> <ref>PMID:8757036</ref> <ref>PMID:9150157</ref> <ref>PMID:9338583</ref> <ref>PMID:9375850</ref> <ref>PMID:9401008</ref> <ref>PMID:9603435</ref> <ref>PMID:14566483</ref> |
| | + | == Function == |
| | + | [https://www.uniprot.org/uniprot/HEXA_HUMAN HEXA_HUMAN] Responsible for the degradation of GM2 gangliosides, and a variety of other molecules containing terminal N-acetyl hexosamines, in the brain and other tissues. The form B is active against certain oligosaccharides. The form S has no measurable activity. |
| | + | == Evolutionary Conservation == |
| | + | [[Image:Consurf_key_small.gif|200px|right]] |
| | + | Check<jmol> |
| | + | <jmolCheckbox> |
| | + | <scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/gk/2gk1_consurf.spt"</scriptWhenChecked> |
| | + | <scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview03.spt</scriptWhenUnchecked> |
| | + | <text>to colour the structure by Evolutionary Conservation</text> |
| | + | </jmolCheckbox> |
| | + | </jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=2gk1 ConSurf]. |
| | + | <div style="clear:both"></div> |
| | + | <div style="background-color:#fffaf0;"> |
| | + | == Publication Abstract from PubMed == |
| | + | Lysosomal beta-hexosaminidase A (Hex A) is essential for the degradation of GM2 gangliosides in the central and peripheral nervous system. Accumulation of GM2 leads to severely debilitating neurodegeneration associated with Tay-Sachs disease (TSD), Sandoff disease (SD) and AB variant. Here, we present the X-ray crystallographic structure of Hex A to 2.8 A resolution and the structure of Hex A in complex with NAG-thiazoline, (NGT) to 3.25 A resolution. NGT, a mechanism-based inhibitor, has been shown to act as a chemical chaperone that, to some extent, prevents misfolding of a Hex A mutant associated with adult onset Tay Sachs disease and, as a result, increases the residual activity of Hex A to a level above the critical threshold for disease. The crystal structure of Hex A reveals an alphabeta heterodimer, with each subunit having a functional active site. Only the alpha-subunit active site can hydrolyze GM2 gangliosides due to a flexible loop structure that is removed post-translationally from beta, and to the presence of alphaAsn423 and alphaArg424. The loop structure is involved in binding the GM2 activator protein, while alphaArg424 is critical for binding the carboxylate group of the N-acetyl-neuraminic acid residue of GM2. The beta-subunit lacks these key residues and has betaAsp452 and betaLeu453 in their place; the beta-subunit therefore cleaves only neutral substrates efficiently. Mutations in the alpha-subunit, associated with TSD, and those in the beta-subunit, associated with SD are discussed. The effect of NGT binding in the active site of a mutant Hex A and its effect on protein function is discussed. |
| | | | |
| - | '''X-ray crystal structure of NGT-bound HexA'''
| + | Crystallographic structure of human beta-hexosaminidase A: interpretation of Tay-Sachs mutations and loss of GM2 ganglioside hydrolysis.,Lemieux MJ, Mark BL, Cherney MM, Withers SG, Mahuran DJ, James MN J Mol Biol. 2006 Jun 16;359(4):913-29. Epub 2006 Apr 27. PMID:16698036<ref>PMID:16698036</ref> |
| | | | |
| | + | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> |
| | + | </div> |
| | + | <div class="pdbe-citations 2gk1" style="background-color:#fffaf0;"></div> |
| | | | |
| - | ==Overview== | + | ==See Also== |
| - | Lysosomal beta-hexosaminidase A (Hex A) is essential for the degradation of GM2 gangliosides in the central and peripheral nervous system. Accumulation of GM2 leads to severely debilitating neurodegeneration associated with Tay-Sachs disease (TSD), Sandoff disease (SD) and AB variant. Here, we present the X-ray crystallographic structure of Hex A to 2.8 A resolution and the structure of Hex A in complex with NAG-thiazoline, (NGT) to 3.25 A resolution. NGT, a mechanism-based inhibitor, has been shown to act as a chemical chaperone that, to some extent, prevents misfolding of a Hex A mutant associated with adult onset Tay Sachs disease and, as a result, increases the residual activity of Hex A to a level above the critical threshold for disease. The crystal structure of Hex A reveals an alphabeta heterodimer, with each subunit having a functional active site. Only the alpha-subunit active site can hydrolyze GM2 gangliosides due to a flexible loop structure that is removed post-translationally from beta, and to the presence of alphaAsn423 and alphaArg424. The loop structure is involved in binding the GM2 activator protein, while alphaArg424 is critical for binding the carboxylate group of the N-acetyl-neuraminic acid residue of GM2. The beta-subunit lacks these key residues and has betaAsp452 and betaLeu453 in their place; the beta-subunit therefore cleaves only neutral substrates efficiently. Mutations in the alpha-subunit, associated with TSD, and those in the beta-subunit, associated with SD are discussed. The effect of NGT binding in the active site of a mutant Hex A and its effect on protein function is discussed.
| + | *[[Beta-Hexosaminidase|Beta-Hexosaminidase]] |
| - | | + | *[[Beta-Hexosaminidase 3D structures|Beta-Hexosaminidase 3D structures]] |
| - | ==About this Structure== | + | *[[Beta-N-acetylhexosaminidase 3D structures|Beta-N-acetylhexosaminidase 3D structures]] |
| - | 2GK1 is a [[Protein complex]] structure of sequences from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2GK1 OCA].
| + | == References == |
| - | | + | <references/> |
| - | ==Reference==
| + | __TOC__ |
| - | Crystallographic structure of human beta-hexosaminidase A: interpretation of Tay-Sachs mutations and loss of GM2 ganglioside hydrolysis., Lemieux MJ, Mark BL, Cherney MM, Withers SG, Mahuran DJ, James MN, J Mol Biol. 2006 Jun 16;359(4):913-29. Epub 2006 Apr 27. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/16698036 16698036]
| + | </StructureSection> |
| - | [[Category: Beta-N-acetylhexosaminidase]]
| + | |
| | [[Category: Homo sapiens]] | | [[Category: Homo sapiens]] |
| - | [[Category: Protein complex]] | + | [[Category: Large Structures]] |
| - | [[Category: Cherney, M M.]] | + | [[Category: Cherney MM]] |
| - | [[Category: James, M N.]] | + | [[Category: James MN]] |
| - | [[Category: Lemieux, M J.]] | + | [[Category: Lemieux MJ]] |
| - | [[Category: Mahuran, D J.]] | + | [[Category: Mahuran DJ]] |
| - | [[Category: Mark, B L.]] | + | [[Category: Mark BL]] |
| - | [[Category: Withers, S G.]] | + | [[Category: Withers SG]] |
| - | [[Category: beta-hexoasaminidase some]]
| + | |
| - | [[Category: glycosidase]]
| + | |
| - | [[Category: gm2 gangliodosis]]
| + | |
| - | [[Category: nag-thazoline]]
| + | |
| - | [[Category: sandhoff disease]]
| + | |
| - | [[Category: tay-sachs disease]]
| + | |
| - | | + | |
| - | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Mon Mar 31 03:17:44 2008''
| + | |
| Structural highlights
Disease
HEXA_HUMAN Defects in HEXA are the cause of GM2-gangliosidosis type 1 (GM2G1) [MIM:272800; also known as Tay-Sachs disease. GM2-gangliosidosis is an autosomal recessive lysosomal storage disease marked by the accumulation of GM2 gangliosides in the neuronal cells. GM2G1 is characterized by GM2 gangliosides accumulation in the absence of HEXA activity, leading to neurodegeneration and, in the infantile form, death in early childhood. GM2G1 has an increased incidence among Ashkenazi Jews and French Canadians in eastern Quebec. It exists in several forms: infantile (most common and most severe), juvenile and adult (late onset).[1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] [13] [14] [15] [16] [17] [18] [19] [20] [21] [22]
Function
HEXA_HUMAN Responsible for the degradation of GM2 gangliosides, and a variety of other molecules containing terminal N-acetyl hexosamines, in the brain and other tissues. The form B is active against certain oligosaccharides. The form S has no measurable activity.
Evolutionary Conservation
Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf.
Publication Abstract from PubMed
Lysosomal beta-hexosaminidase A (Hex A) is essential for the degradation of GM2 gangliosides in the central and peripheral nervous system. Accumulation of GM2 leads to severely debilitating neurodegeneration associated with Tay-Sachs disease (TSD), Sandoff disease (SD) and AB variant. Here, we present the X-ray crystallographic structure of Hex A to 2.8 A resolution and the structure of Hex A in complex with NAG-thiazoline, (NGT) to 3.25 A resolution. NGT, a mechanism-based inhibitor, has been shown to act as a chemical chaperone that, to some extent, prevents misfolding of a Hex A mutant associated with adult onset Tay Sachs disease and, as a result, increases the residual activity of Hex A to a level above the critical threshold for disease. The crystal structure of Hex A reveals an alphabeta heterodimer, with each subunit having a functional active site. Only the alpha-subunit active site can hydrolyze GM2 gangliosides due to a flexible loop structure that is removed post-translationally from beta, and to the presence of alphaAsn423 and alphaArg424. The loop structure is involved in binding the GM2 activator protein, while alphaArg424 is critical for binding the carboxylate group of the N-acetyl-neuraminic acid residue of GM2. The beta-subunit lacks these key residues and has betaAsp452 and betaLeu453 in their place; the beta-subunit therefore cleaves only neutral substrates efficiently. Mutations in the alpha-subunit, associated with TSD, and those in the beta-subunit, associated with SD are discussed. The effect of NGT binding in the active site of a mutant Hex A and its effect on protein function is discussed.
Crystallographic structure of human beta-hexosaminidase A: interpretation of Tay-Sachs mutations and loss of GM2 ganglioside hydrolysis.,Lemieux MJ, Mark BL, Cherney MM, Withers SG, Mahuran DJ, James MN J Mol Biol. 2006 Jun 16;359(4):913-29. Epub 2006 Apr 27. PMID:16698036[23]
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
See Also
References
- ↑ Nakano T, Muscillo M, Ohno K, Hoffman AJ, Suzuki K. A point mutation in the coding sequence of the beta-hexosaminidase alpha gene results in defective processing of the enzyme protein in an unusual GM2-gangliosidosis variant. J Neurochem. 1988 Sep;51(3):984-7. PMID:2970528
- ↑ Navon R, Proia RL. The mutations in Ashkenazi Jews with adult GM2 gangliosidosis, the adult form of Tay-Sachs disease. Science. 1989 Mar 17;243(4897):1471-4. PMID:2522679
- ↑ Tanaka A, Punnett HH, Suzuki K. A new point mutation in the beta-hexosaminidase alpha subunit gene responsible for infantile Tay-Sachs disease in a non-Jewish Caucasian patient (a Kpn mutant). Am J Hum Genet. 1990 Sep;47(3):568-74. PMID:2144098
- ↑ Akli S, Chelly J, Lacorte JM, Poenaru L, Kahn A. Seven novel Tay-Sachs mutations detected by chemical mismatch cleavage of PCR-amplified cDNA fragments. Genomics. 1991 Sep;11(1):124-34. PMID:1837283
- ↑ Mules EH, Hayflick S, Miller CS, Reynolds LW, Thomas GH. Six novel deleterious and three neutral mutations in the gene encoding the alpha-subunit of hexosaminidase A in non-Jewish individuals. Am J Hum Genet. 1992 Apr;50(4):834-41. PMID:1532289
- ↑ Fernandes M, Kaplan F, Natowicz M, Prence E, Kolodny E, Kaback M, Hechtman P. A new Tay-Sachs disease B1 allele in exon 7 in two compound heterozygotes each with a second novel mutation. Hum Mol Genet. 1992 Dec;1(9):759-61. PMID:1302612
- ↑ Trop I, Kaplan F, Brown C, Mahuran D, Hechtman P. A glycine250--> aspartate substitution in the alpha-subunit of hexosaminidase A causes juvenile-onset Tay-Sachs disease in a Lebanese-Canadian family. Hum Mutat. 1992;1(1):35-9. PMID:1301189 doi:http://dx.doi.org/10.1002/humu.1380010106
- ↑ Akalin N, Shi HP, Vavougios G, Hechtman P, Lo W, Scriver CR, Mahuran D, Kaplan F. Novel Tay-Sachs disease mutations from China. Hum Mutat. 1992;1(1):40-6. PMID:1301190 doi:http://dx.doi.org/10.1002/humu.1380010107
- ↑ Akli S, Chomel JC, Lacorte JM, Bachner L, Kahn A, Poenaru L. Ten novel mutations in the HEXA gene in non-Jewish Tay-Sachs patients. Hum Mol Genet. 1993 Jan;2(1):61-7. PMID:8490625
- ↑ Harmon DL, Gardner-Medwin D, Stirling JL. Two new mutations in a late infantile Tay-Sachs patient are both in exon 1 of the beta-hexosaminidase alpha subunit gene. J Med Genet. 1993 Feb;30(2):123-8. PMID:8445615
- ↑ Tomczak J, Grebner EE. Three novel beta-hexosaminidase A mutations in obligate carriers of Tay-Sachs disease. Hum Mutat. 1994;4(1):71-2. PMID:7951261 doi:http://dx.doi.org/10.1002/humu.1380040112
- ↑ Tanaka A, Sakazaki H, Murakami H, Isshiki G, Suzuki K. Molecular genetics of Tay-Sachs disease in Japan. J Inherit Metab Dis. 1994;17(5):593-600. PMID:7837766
- ↑ Triggs-Raine B, Richard M, Wasel N, Prence EM, Natowicz MR. Mutational analyses of Tay-Sachs disease: studies on Tay-Sachs carriers of French Canadian background living in New England. Am J Hum Genet. 1995 Apr;56(4):870-9. PMID:7717398
- ↑ Peleg L, Meltzer F, Karpati M, Goldman B. GM2 gangliosidosis B1 variant: biochemical and molecular characterization of hexosaminidase A. Biochem Mol Med. 1995 Apr;54(2):126-32. PMID:8581357
- ↑ Navon R, Khosravi R, Korczyn T, Masson M, Sonnino S, Fardeau M, Eymard B, Lefevre M, Turpin JC, Rondot P, et al.. A new mutation in the HEXA gene associated with a spinal muscular atrophy phenotype. Neurology. 1995 Mar;45(3 Pt 1):539-43. PMID:7898712
- ↑ De Gasperi R, Gama Sosa MA, Battistini S, Yeretsian J, Raghavan S, Zelnik N, Leshinsky E, Kolodny EH. Late-onset GM2 gangliosidosis: Ashkenazi Jewish family with an exon 5 mutation (Tyr180-->His) in the Hex A alpha-chain gene. Neurology. 1996 Aug;47(2):547-52. PMID:8757036
- ↑ Akerman BR, Natowicz MR, Kaback MM, Loyer M, Campeau E, Gravel RA. Novel mutations and DNA-based screening in non-Jewish carriers of Tay-Sachs disease. Am J Hum Genet. 1997 May;60(5):1099-106. PMID:9150157
- ↑ Kaufman M, Grinshpun-Cohen J, Karpati M, Peleg L, Goldman B, Akstein E, Adam A, Navon R. Tay-Sachs disease and HEXA mutations among Moroccan Jews. Hum Mutat. 1997;10(4):295-300. PMID:9338583 doi:<295::AID-HUMU5>3.0.CO;2-G 10.1002/(SICI)1098-1004(1997)10:4<295::AID-HUMU5>3.0.CO;2-G
- ↑ Gil Ribeiro M, Pinto RA, Suzuki K, Sa Miranda MC. Two novel (1334delC and 1363G to A, G455R) mutations in exon 12 of the beta-hexosaminidase alpha-chain gene in two Portuguese patients. Hum Mutat. 1997;10(5):359-60. PMID:9375850 doi:<359::AID-HUMU4>3.0.CO;2-A 10.1002/(SICI)1098-1004(1997)10:5<359::AID-HUMU4>3.0.CO;2-A
- ↑ Drucker L, Hemli JA, Navon R. Two mutated HEXA alleles in a Druze patient with late-infantile Tay-Sachs disease. Hum Mutat. 1997;10(6):451-7. PMID:9401008 doi:<451::AID-HUMU6>3.0.CO;2-G 10.1002/(SICI)1098-1004(1997)10:6<451::AID-HUMU6>3.0.CO;2-G
- ↑ Petroulakis E, Cao Z, Clarke JT, Mahuran DJ, Lee G, Triggs-Raine B. W474C amino acid substitution affects early processing of the alpha-subunit of beta-hexosaminidase A and is associated with subacute G(M2) gangliosidosis. Hum Mutat. 1998;11(6):432-42. PMID:9603435 doi:<432::AID-HUMU3>3.0.CO;2-Z 10.1002/(SICI)1098-1004(1998)11:6<432::AID-HUMU3>3.0.CO;2-Z
- ↑ Tanaka A, Hoang LT, Nishi Y, Maniwa S, Oka M, Yamano T. Different attenuated phenotypes of GM2 gangliosidosis variant B in Japanese patients with HEXA mutations at codon 499, and five novel mutations responsible for infantile acute form. J Hum Genet. 2003;48(11):571-4. Epub 2003 Oct 18. PMID:14566483 doi:10.1007/s10038-003-0080-9
- ↑ Lemieux MJ, Mark BL, Cherney MM, Withers SG, Mahuran DJ, James MN. Crystallographic structure of human beta-hexosaminidase A: interpretation of Tay-Sachs mutations and loss of GM2 ganglioside hydrolysis. J Mol Biol. 2006 Jun 16;359(4):913-29. Epub 2006 Apr 27. PMID:16698036 doi:http://dx.doi.org/10.1016/j.jmb.2006.04.004
|