We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox GGC7
From Proteopedia
(Difference between revisions)
| (112 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | == | + | ==Iduronate 2-sulfatase== |
| - | <StructureSection load=' | + | <StructureSection load='5FQL' size='340' side='right' caption='Iduronate 2-sulfatase protein' scene=''> |
| - | + | Iduronate 2-sulfatase (IDS), also referred to as Alpha-L-iduronate sulfate sulfatase or Idursulfase, is a lysosomal enzyme involved in the degradation pathway of dermatan sulfate and heparan sulfate.[1] | |
| - | + | ||
| - | + | ||
| - | + | ||
== Function == | == Function == | ||
| - | + | Iduronate 2-sulfatase is located in the lysosome.[1] It is involved in the lysosomal degradation pathway of dermatan sulfate and heparan sulfate.[1] IDS hydrolyzes the 2-sulfate groups of the L-iduronate 2-sulfate units of dermatan sulfate, heparan sulfate and heparin.[1] Dermatan sulfate and heparan sulfate are complex glycosaminoglycans, which are essentially large sugar molecules.[2] They play important roles in cell adhesion, growth, proliferation and repair, and their degradation and recycling in the lysosome are essential for cellular maintenance.[2] IDS is expressed in the tissues of the liver, kidney, lung, and placenta.[1] | |
| - | + | ||
| - | + | ||
| - | + | ||
== Disease == | == Disease == | ||
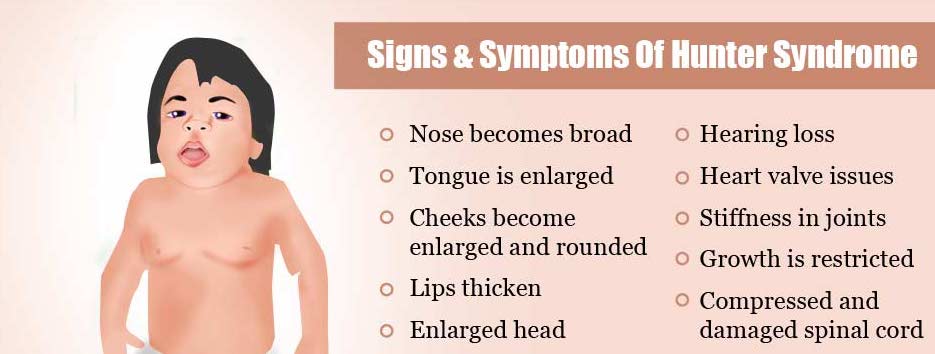
| + | Mutations in Iduronate 2-sulfatase on the Xq28 chromosome can lead to Mucopolysaccharidosis 2 (MPS2), more commonly known as Hunter syndrome.[1] MPS2 is an X-linked lysosomal storage disease.[1] Due to the loss of IDS activity, the disease is characterized by the intracellular accumulation of the glycosaminoglycans heparan sulfate and dermatan sulfate, which are then excreted in urine.[1] Scientists have identified over 500 mutations on the Xq28 chromosome that include rearrangements, insertions/deletions, splicing defects and nonsense point mutations.[2] It is rare to find adults with severe Hunter syndrome as the average life expectancy for those with MPS2 is 15 years of age.[1] Most children diagnosed with MPS2 have somatic abnormalities including skeletal deformities, hepatosplenomegaly, and progressive cardiopulmonary deterioration.[1] Neurological damage is also prevalent beginning with what seems to be a developmental delay and hyperactivity, but progresses to mental retardation and dementia.[1] Death from MPS2 is typically due to obstructive airway disease or cardiac failure.[1] A treatment for patients with mild Hunter syndrome is enzyme replacement therapy, which involves the recombinant human IDS.[2] | ||
| + | [[Image:Signs-and-symptoms-of-hunter-syndrome.jpg]] | ||
| + | == Structural highlights == | ||
| + | <scene name='75/752270/Ide_mutations/1'>Scene 1: Location of mutations</scene> | ||
| - | = | + | <scene name='75/752270/Ide_atp_binding-active_sites/1'>Scene 2: ATP binding active sites</scene> |
| - | + | ||
| - | + | ||
| - | + | <scene name='75/752270/Ide_n_and_c_terminals/1'>Scene 3: N and C terminals</scene> | |
| + | <scene name='75/752270/Active_site_on_chain_a/1'>Scene 4: Active sites</scene> | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
| - | + | 1. UniProt ConsortiumEuropean Bioinformatics InstituteProtein Information ResourceSIB Swiss Institute of Bioinformatics. Iduronate 2-sulfatase https://www.uniprot.org/uniprot/P22304#pathology_and_biotech (accessed Apr 28, 2021). | |
| + | 2. Demydchuk M, Hill CH, Zhou A, Bunkóczi G, Stein PE, Marchesan D, Deane JE, Read RJ. Insights into Hunter syndrome from the structure of iduronate-2-sulfatase. Nat Commun. 2017 Jun 8;8:15786. doi: 10.1038/ncomms15786. PMID: 28593992; PMCID: PMC5472762. | ||
Current revision
Iduronate 2-sulfatase
| |||||||||||
References
1. UniProt ConsortiumEuropean Bioinformatics InstituteProtein Information ResourceSIB Swiss Institute of Bioinformatics. Iduronate 2-sulfatase https://www.uniprot.org/uniprot/P22304#pathology_and_biotech (accessed Apr 28, 2021). 2. Demydchuk M, Hill CH, Zhou A, Bunkóczi G, Stein PE, Marchesan D, Deane JE, Read RJ. Insights into Hunter syndrome from the structure of iduronate-2-sulfatase. Nat Commun. 2017 Jun 8;8:15786. doi: 10.1038/ncomms15786. PMID: 28593992; PMCID: PMC5472762.