User:Khadar Abdi/Sandbox1
From Proteopedia
< User:Khadar Abdi(Difference between revisions)
| (7 intermediate revisions not shown.) | |||
| Line 3: | Line 3: | ||
== Introduction == | == Introduction == | ||
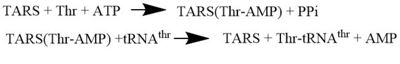
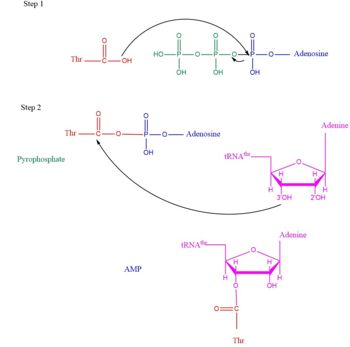
| - | '''Threonyl t-RNA Synthetase''' or '''Threonyl-tRNA ligase''' or '''TARS''' is a homodimer (150kDa in bacteria and 170kDa in human) and is classified as a class II '''Aminoacyl-tRNA synthetase''' enzymes. These ancient enzymes primary function are to add the respective amino acid to the respective transfer Ribonucleic Acid (tRNA-AA), a necessity prep for the protein synthesis pathway<ref>PMID:29305884</ref>. As the name implies, TARS function is to add Threonine amino acid (Thr) to threonine specific tRNA (tRNA-thr) in the presence of Adenosine triphosphate (ATP) and diavalent metal cation. Below displays the overview of TARS Aminoacylation rxn<ref>PMID:29305884</ref>.[[Image:TARS_protein_rxn.jpeg |center|thumb|400px| '''Overall TARS protein rxn. Substrates includes ATP, Thr and tRNA-thr.''']] | + | <scene name='78/786634/Staph_aureus_tars/1'>'''Threonyl t-RNA Synthetase''' or '''Threonyl-tRNA ligase''' or '''TARS'''</scene> is a homodimer (150kDa in bacteria and 170kDa in human) and is classified as a class II '''Aminoacyl-tRNA synthetase''' enzymes. These ancient enzymes primary function are to add the respective amino acid to the respective transfer Ribonucleic Acid (tRNA-AA), a necessity prep for the protein synthesis pathway<ref>PMID:29305884</ref>. As the name implies, TARS function is to add Threonine amino acid (Thr) to threonine specific tRNA (tRNA-thr) in the presence of Adenosine triphosphate (ATP) and diavalent metal cation. Below displays the overview of TARS Aminoacylation rxn<ref>PMID:29305884</ref>.[[Image:TARS_protein_rxn.jpeg |center|thumb|400px| '''Overall TARS protein rxn. Substrates includes ATP, Thr and tRNA-thr.''']] |
Although there are multiple forms of TARS in different cells, this protopedia page mainly focuses on the structure of ''Staphylococcus aureus'' (''S. aureus'') and ''Escherichia coli'' (''E. coli'') cytoplasmic TARS as their is more crystal structures that identify their domains within the protein. | Although there are multiple forms of TARS in different cells, this protopedia page mainly focuses on the structure of ''Staphylococcus aureus'' (''S. aureus'') and ''Escherichia coli'' (''E. coli'') cytoplasmic TARS as their is more crystal structures that identify their domains within the protein. | ||
| Line 21: | Line 21: | ||
The <scene name='78/786634/Tgs-domain/2'>N1-Domain</scene> and <scene name='78/786634/Editing_domain/1'>N-2 domain</scene> is the found in the N-terminus of the TARS enzyme chain. Specific N1 is found from 1-62 residue while N2 domain is found from 63-241 residue(PDB: [[1tje]]). Although located in neighboring regions, the N1-domain and N2-domain deviate dramatically from one another. Structurally, N1-domain has a TGS-fold (short for TARS, GTPase, Spo1 domain as their the most common proteins that present this fold)<ref>PMID:10447505</ref> which has 3 antiparallel beta sheets interacting with an alpha helix causing a formation of roll-like structure (similar to the description by Cath: Ubiquitin-like roll)<ref>http://www.rcsb.org/pdb/explore/macroMoleculeData.do?structureId=1TJE</ref>. The structure has no known function but it is pronounced structure found common within TARS. | The <scene name='78/786634/Tgs-domain/2'>N1-Domain</scene> and <scene name='78/786634/Editing_domain/1'>N-2 domain</scene> is the found in the N-terminus of the TARS enzyme chain. Specific N1 is found from 1-62 residue while N2 domain is found from 63-241 residue(PDB: [[1tje]]). Although located in neighboring regions, the N1-domain and N2-domain deviate dramatically from one another. Structurally, N1-domain has a TGS-fold (short for TARS, GTPase, Spo1 domain as their the most common proteins that present this fold)<ref>PMID:10447505</ref> which has 3 antiparallel beta sheets interacting with an alpha helix causing a formation of roll-like structure (similar to the description by Cath: Ubiquitin-like roll)<ref>http://www.rcsb.org/pdb/explore/macroMoleculeData.do?structureId=1TJE</ref>. The structure has no known function but it is pronounced structure found common within TARS. | ||
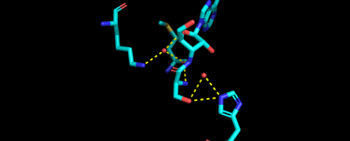
| - | This is the opposite of the N2-domain, or sometimes called TARS additional domain. This is ironic as this addition is very important for proofreading TARS activity<ref>PMID:15525511</ref>. TARS N2-domain is described as 2-layer alpha-beta sandwich comprised of mostly alpha helices. Interesting enough, the superfamily of this motif is found to be TARS and Alanyl-tRNA synthetase (AARS)common domain. This domain function similar by discriminating Threonine and Alanine from Serine amino acid (Ser) as serine is similar structure to both amino acids. The editing domain functions first by moving Ser bound tRNA-Thr from the catalytic domain to the <scene name='78/786634/Editing_domain_residues/1'>editing site</scene> by breaking the bonds nucleotide A73, C74, and C75 from the catalytic site allowing the acceptor arm of tRNA-Thr to flip to the editing domain of S12-S13 site. Research on editing hydrolysis propose that residue Tyr 103 plays an important role of guiding acceptor arm towards the editing domain. The Ser bound to tRNA is | + | This is the opposite of the N2-domain, or sometimes called TARS additional domain. This is ironic as this addition is very important for proofreading TARS activity<ref>PMID:15525511</ref>. TARS N2-domain is described as 2-layer alpha-beta sandwich comprised of mostly alpha helices. Interesting enough, the superfamily of this motif is found to be TARS and Alanyl-tRNA synthetase (AARS)common domain. This domain function similar by discriminating Threonine and Alanine from Serine amino acid (Ser) as serine is similar structure to both amino acids. The editing domain functions first by moving Ser bound tRNA-Thr from the catalytic domain to the <scene name='78/786634/Editing_domain_residues/1'>editing site</scene> by breaking the bonds nucleotide A73, C74, and C75 from the catalytic site allowing the acceptor arm of tRNA-Thr to flip to the editing domain of S12-S13 site. Research on editing hydrolysis propose that residue Tyr 103 plays an important role of guiding acceptor arm towards the editing domain. The Ser bound to tRNA is hydrolyze by a water molecule, interacting with His73, acting as a nucleophile to the alpha carbon of serine, follow by protonation by a 2nd water molecule interacting with carbonyl of Met181 and amide side chain Lys156. The specificity of serine binding to editing site versus threonine is due to steric hindrance by the presents of methyl group in the side chain. What is amazing about the fidility mechanism of AA-tRNA binding is the domain is much similar to (AARS) found in the C-terminus as they share 40% similarity in residues and both select for serine, although AARS also . The subtle difference of the isoforms mitochondrial TARS from cytoplasmic TARS appears in editing as the mitochondrial TARS doesn't have N2-domain and requires interaction to hydrolyze serine <ref>PMID:22773845</ref>. |
| - | [[Image:Editing domain.png|center|thumb|350px|'''Representative image of editing domain that demonstrates amino acid important in binding and hydrolyzing serine-tRNA(thr). Note, the amino acids highlighted in light blue are the amino acid important for hydrolysis (His-73, Met181, and Lys 156). Also not shown is Tyr103 who plays an important role in changing tRNA acceptor stem from catalytic domain to editing domain.''']] | + | [[Image:Editing domain new.png|center|thumb|350px|'''Representative image of editing domain that demonstrates amino acid important in binding and hydrolyzing serine-tRNA(thr). Note, the amino acids highlighted in light blue are the amino acid important for hydrolysis (His-73, Met181, and Lys 156). Also not shown is Tyr103 who plays an important role in changing tRNA acceptor stem from catalytic domain to editing domain.''']] |
| + | |||
| + | Although separated, both the N2-domain and catalytic domain play an <scene name='78/786634/E_coli_tars_binding_to_trna/1'>important role of binding the tRNA acceptor stem to TARS.</scene> | ||
===Catalytic Domain (Residue 243-532)=== | ===Catalytic Domain (Residue 243-532)=== | ||
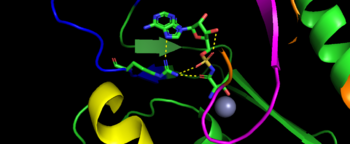
| - | As the name implies, the catalytic domain is the main area of | + | As the name implies, the <scene name='78/786634/Catalytic_structure_of_tars/1'>catalytic domain</scene> is the main area of aminoacylation activity. This domain is linked with N2-Domain of TARS by a alpha helix, or a linker helix (residue 225-242)<ref>PMID:10319817</ref>. The domain is architecturally described as a 2-layer alpha beta sandwhich. The importance of the domain is that it has 3 sites of binding: motif 1: ordering loop; motif 2 loop, Motif 3: threonine loop, and motif 4: ATP loop. The main important residues and cofactors within in the catalytic site include: Tyr468, Arg365, His309, Zinc and Magnesium. |
| + | [[Image:Catalytic domain motif.png|center|thumb|350px|'''Image represents the motif of ''S. Aureus'' TARS. Motif 1 is represent in yellow, motif 2 is represented in blue, motif 3 is represented in orange, and motif 4 is magenta]] | ||
| - | + | The binding pocket for threonine consistent of Arg363 found in motif 2, Tyr462 found in threonine loop, and zinc divalent ion (Zn2+) coordinating by his-his-cys residues and a water molecule (for ''S. aureus'' it was Cys336, His387, and His571; versus ''E. coli.'' was Cys334, His385, and His511, thus preventing similar structure molecules that lack hydroxyl group from binding such as valine. The presence of Thr amino acid causes a movement of Tyr468 to move closer to the ordering loop, while the Arg363 is displace to have more interactions with threonine, as well as bind to the alpha phosphate of ATP. Asides Arg363, Glu365 and Arg 375 bind to the gamma and beta phosphates of ATP to stabilize the pyrophosphate product, however both of these residues are not as well conserved as Arg363. The adenine ring is also stabilize in the presence of motif 3 argnine and Phe379. In the presence of Mg2+ (to stabilize the product form), the adenylation reaction occurs, which breaks the bounds seen in Glu365 and Arg 375 to phosphates and the whole ATP loop moving. | |
| + | |||
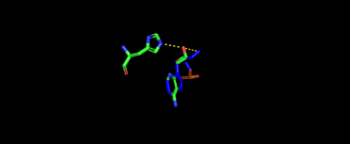
| + | [[Image:S.Aureus binding to threonyl-adenylate.png|center|thumb|350px|'''Representative image of catalytic domain binding of threonyl-adenylate. The image includes residue Arg365 binding to threonyl-adenylate representing ATP and Thr amino acylation activity.]] | ||
| + | |||
| + | The 2nd step, formation of thr-tRNA-thr, occurs through tRNA binding to anticodon domain (which is discussed later on) shifting threonine loop and ATP loops, an thus bound threonyl-adenylate, towards the tRNA. The terminal 3'adenine is then hydrogen bound to Tyr462 to stabilize the molecule, follow by subjected to acid-base reaction at the 2'OH site with His309 to catalyze aminoacyl-transfer reaction<ref>PMID:18997014</ref>. | ||
| + | |||
| + | [[Image:E.coli. binding to tRNA.png|center|thumb|350px|'''Representative image of catalytic domain binding of tRNA. The image includes residue His309 binding to tRNA 3'adenine representing acid-base reaction with 2'OH.]] | ||
===Anti-Codon Domain(Residue 533-645)=== | ===Anti-Codon Domain(Residue 533-645)=== | ||
| - | Lastly, there is the C-terminus domain of the TARS protein, otherwise known as the anticodon-domain. This alpha-beta region is conserved through most aaRs class 2 molecules but have a specificity towards binding to tRNA-thr. This domain has a Rossmann-fold, which is 3-layer sandwhich fold necessary for binding of nucleotides, in this case tRNA nucleotides. This domain recognize and bind to tRNA-thr major groove containing BGU anticodon consensus sequence (B: Guanine, Cytosine, or Uracil). Although tRNA binding is important to hold on for aminoacylation, this should not be confused with having any aminoacylation activity. | + | Lastly, there is the C-terminus domain of the TARS protein, otherwise known as the <scene name='78/786634/E_coli_tars_binding_to_trna/4'>anticodon-domain</scene>. This alpha-beta region is conserved through most aaRs class 2 molecules but have a specificity towards binding to tRNA-thr. This domain has a Rossmann-fold, which is 3-layer sandwhich fold necessary for binding of nucleotides, in this case tRNA nucleotides. This domain recognize and bind to tRNA-thr major groove containing BGU anticodon consensus sequence (B: Guanine, Cytosine, or Uracil). Although tRNA binding is important to hold on for aminoacylation, this should not be confused with having any aminoacylation activity. |
| + | |||
| + | [[Image:S.Aureus binding to threonyl-adenylate.png|center|thumb|350px|'''Representative image of catalytic domain binding of threonyl-adenylate. The image includes residue Arg365 binding to threonyl-adenylate representing ATP and Thr amino acylation activity.]] | ||
| Line 47: | Line 58: | ||
Lately the aaRS family was found to have more function than just aminoacylation. For instance, many aaRs molecules have been found to link with angiogenesis, blood vessel growth occuring within a cancer environment<ref>PMID:25535072</ref>. This is seen in human exogenous TARS in its ability to generate blood vessels within ovarian cancer environment<ref>PMID:23425968</ref>. Studies on the structure of TARS bound to BC194, derivative to the natural antibiotic Borrelidin, were investigated to understand how the angiogenic signaling from TARS occurs<ref>PMID: 26271225</ref>. | Lately the aaRS family was found to have more function than just aminoacylation. For instance, many aaRs molecules have been found to link with angiogenesis, blood vessel growth occuring within a cancer environment<ref>PMID:25535072</ref>. This is seen in human exogenous TARS in its ability to generate blood vessels within ovarian cancer environment<ref>PMID:23425968</ref>. Studies on the structure of TARS bound to BC194, derivative to the natural antibiotic Borrelidin, were investigated to understand how the angiogenic signaling from TARS occurs<ref>PMID: 26271225</ref>. | ||
| - | [[Image:Borrelidin binding.jpg|center|thumb|500px| '''Representative image of human TARS binding sites of BC194, TARS inhibitor. These sites show potential areas of where angiogenic activity of TARS. Note residue H388 and R442, amino acids that play important role catalytic activity, were found to interact with BC194, showing a potential of aminoacylation activity to possibly interconnect with angiogeneic activity. Not shown is TARS conformation change after presence of BC194 also a possible mechanism of inhibiting angiogeneic activity.''' <ref>PMID:26271225</ref>]] | ||
== List to available structures == | == List to available structures == | ||
Current revision
Threonyl-tRNA Synthetase/ligase
| |||||||||||